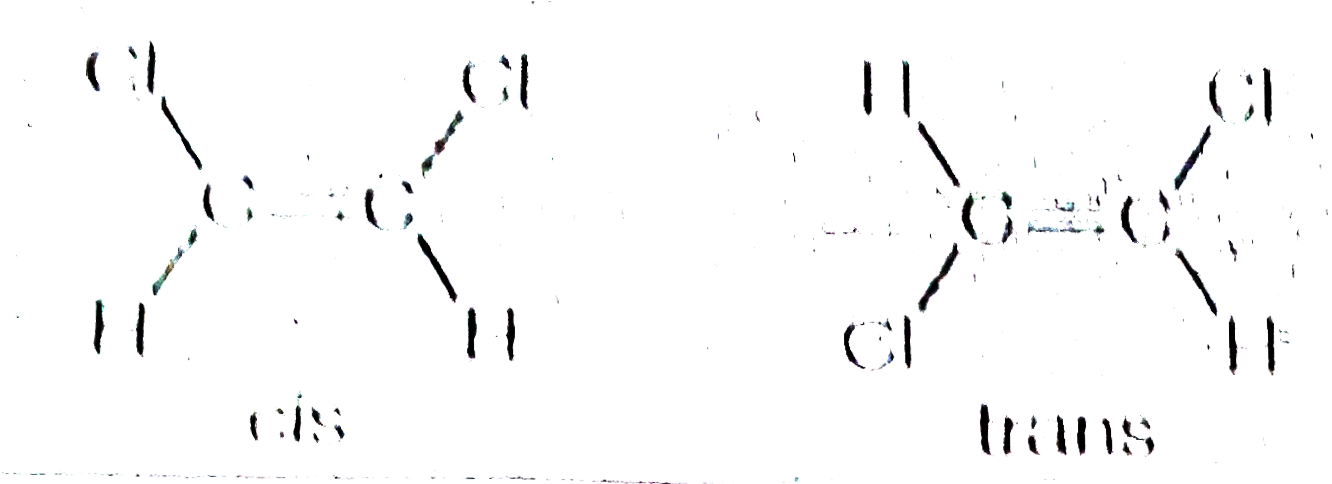Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which isomer will have a higher boiling point?
Text Solution
|
- The element having the higher boiling point is
Text Solution
|
- Assertion : Boiling points of cis-isomers are higher than trans - isom...
Text Solution
|
- Assertion. Boiling points of cis-isomers are higher than those of tran...
Text Solution
|
- Which isomer is expected to have a higher melting point?
Text Solution
|
- Which isomer will have a higher boiling point?
Text Solution
|
- Assertion : Boiling points of cis-isomers are higher than trans - isom...
Text Solution
|
- कीटोन का क्वंथनाक संगत समावयवी ऐल्डिहाइड की अपेक्षा कुछ अधिक क्यों होत...
Text Solution
|
- Assertion (A) : The boiling point of straight chain isomers have ...
Text Solution
|