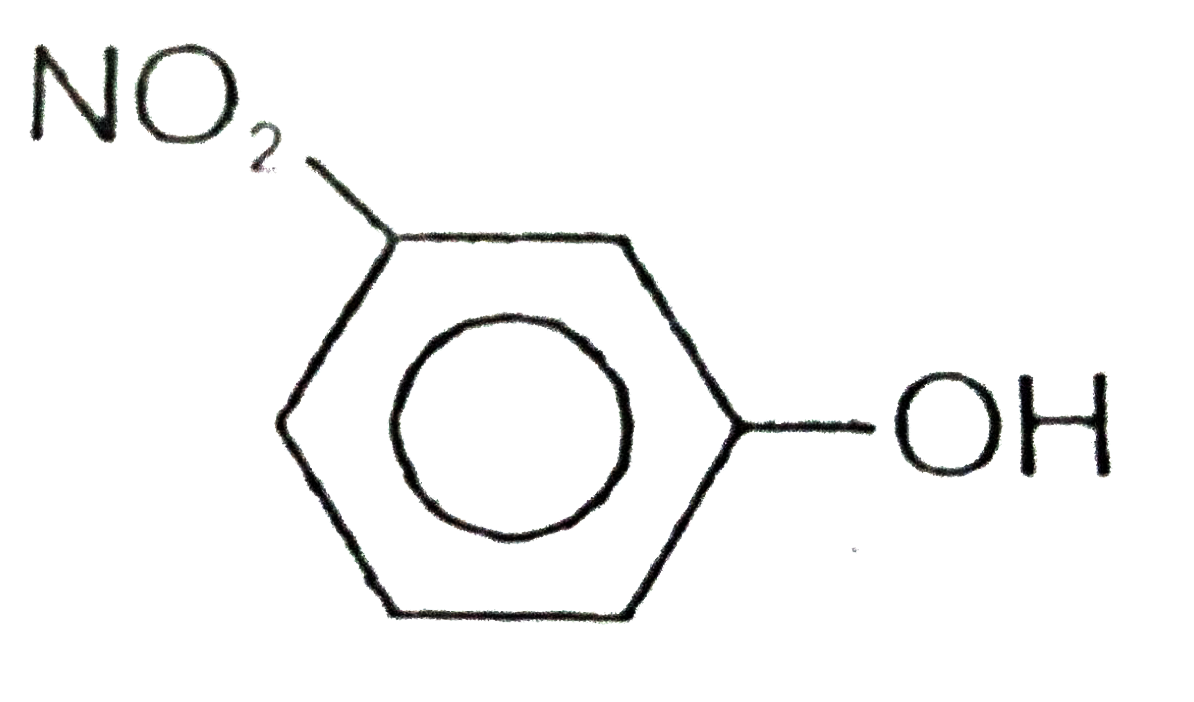
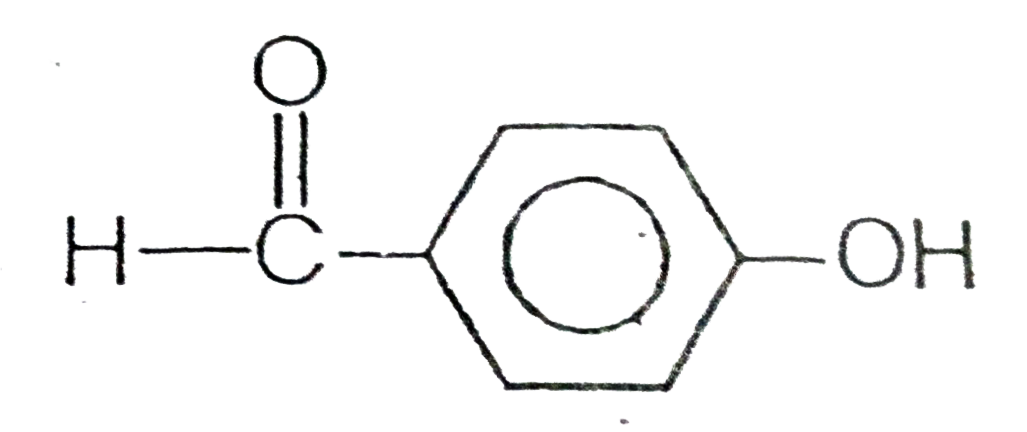
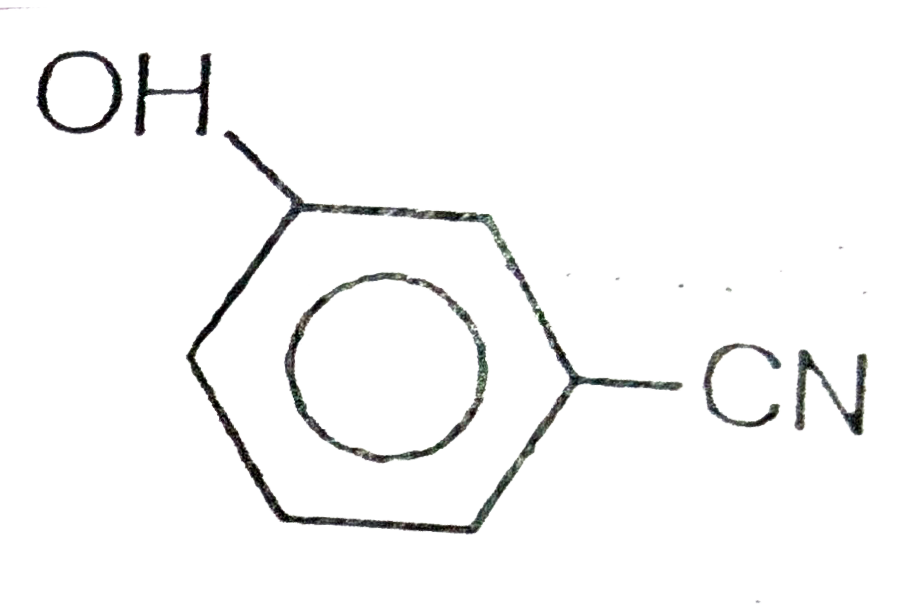
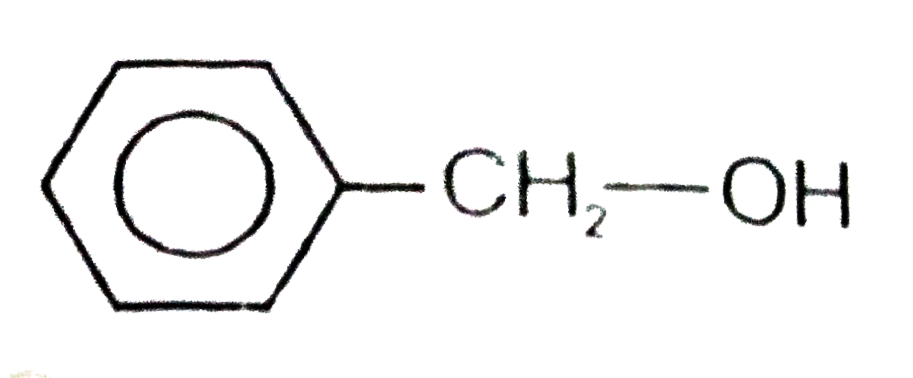A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one is phenol ?
Text Solution
|
- Amongst the following phenols which one is most acidic ?
Text Solution
|
- Which one of the following properties is exhibited by phenol ?
Text Solution
|
- Which one is phenol ?
Text Solution
|
- One percent phenol is
Text Solution
|
- Which one of the following phenols will show highest acidity?
Text Solution
|
- Which one is not a resonance form of a phenolate ion?
Text Solution
|
- Which one of the following phenols has the highestpK value?
Text Solution
|
- When phenol reacts with which one of the following reagents ,a conjuga...
Text Solution
|