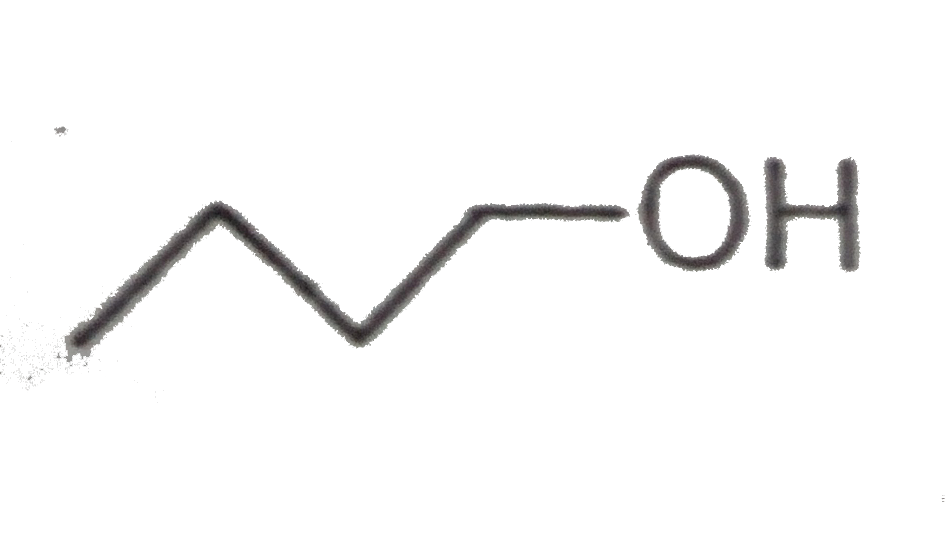
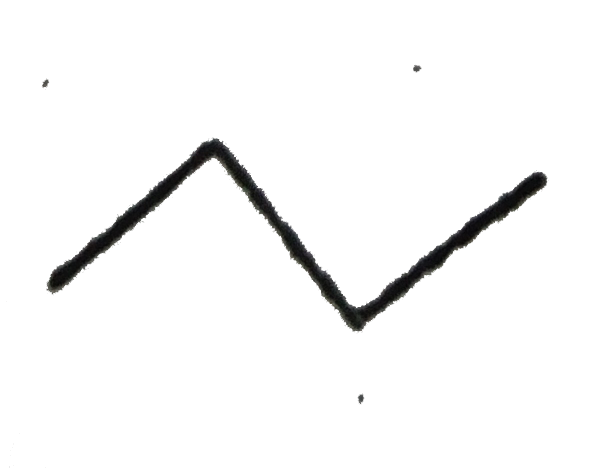
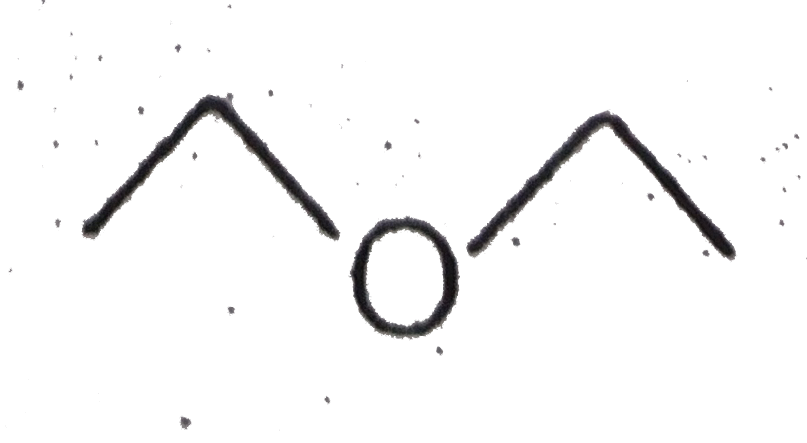
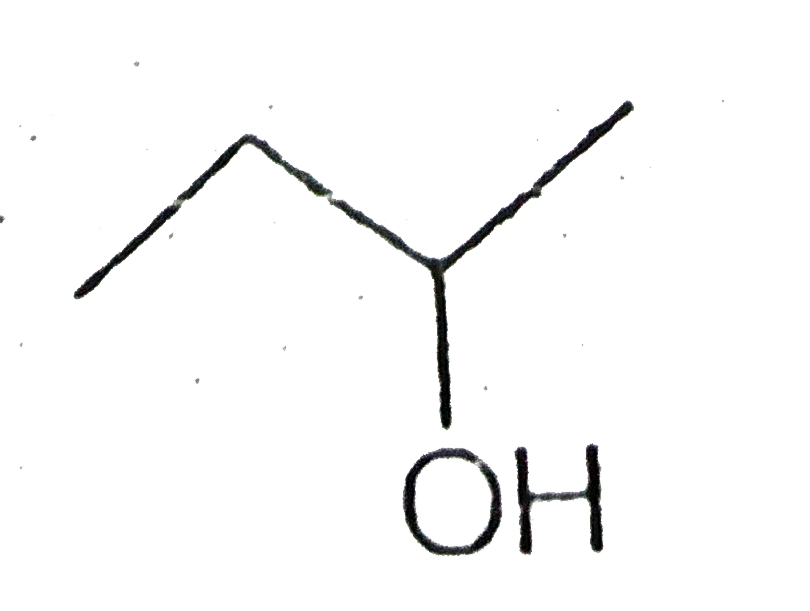
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Boiling point will be least for
Text Solution
|
- Which of the following has least boiling point ?
Text Solution
|
- Name the following : noble gas with least boiling point
Text Solution
|
- Which of the following alkyl chloride will have the least boiling poin...
Text Solution
|
- Which of the following has the least boiling point ?
Text Solution
|
- Which of the following has least boiling point ?
Text Solution
|
- Boiling point is least for
Text Solution
|
- Which of the following has the least boiling point ?
Text Solution
|
- Boiling point is least for
Text Solution
|