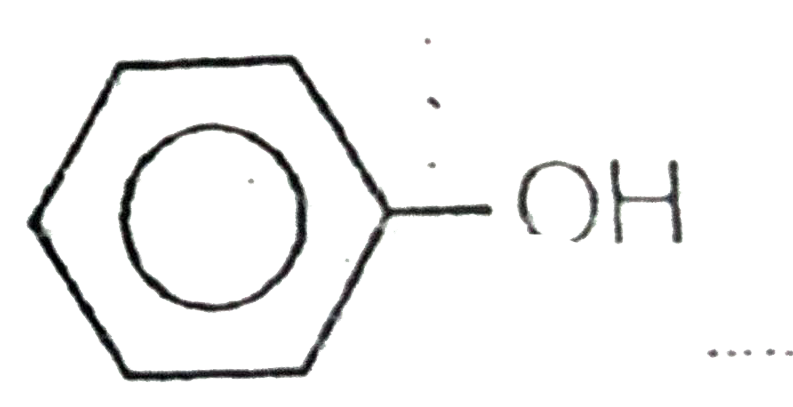
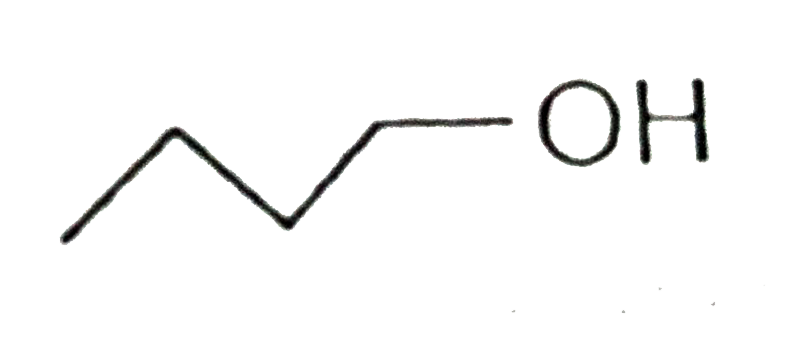
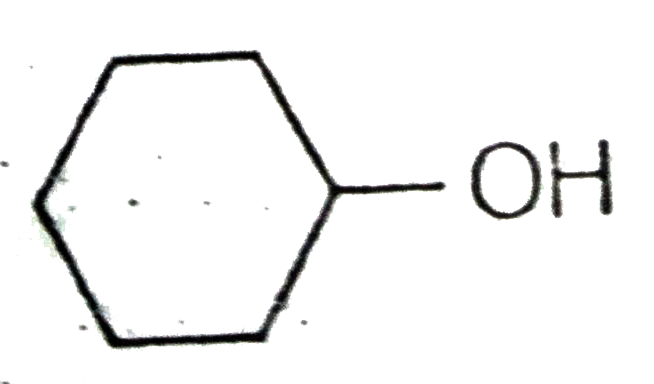
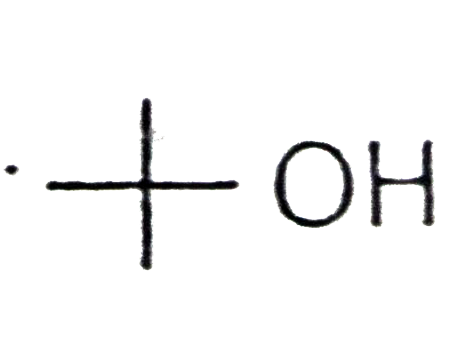A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Molecule which does not oxidise
Text Solution
|
- Which molecule does not exist ?
Text Solution
|
- In which of the following reactions does reactions does water act as o...
Text Solution
|
- Which of the following molecules can act as an oxidising agent as well...
Text Solution
|
- Which of the following molecules can act as an oxidising as well as a ...
Text Solution
|
- Molecule which does not oxidise
Text Solution
|
- Ozone does not oxidise which one of the following
Text Solution
|
- Which reaction does not have the correct oxidising agent ?
Text Solution
|
- Which of the following molecules can act as an oxidating as well as a ...
Text Solution
|