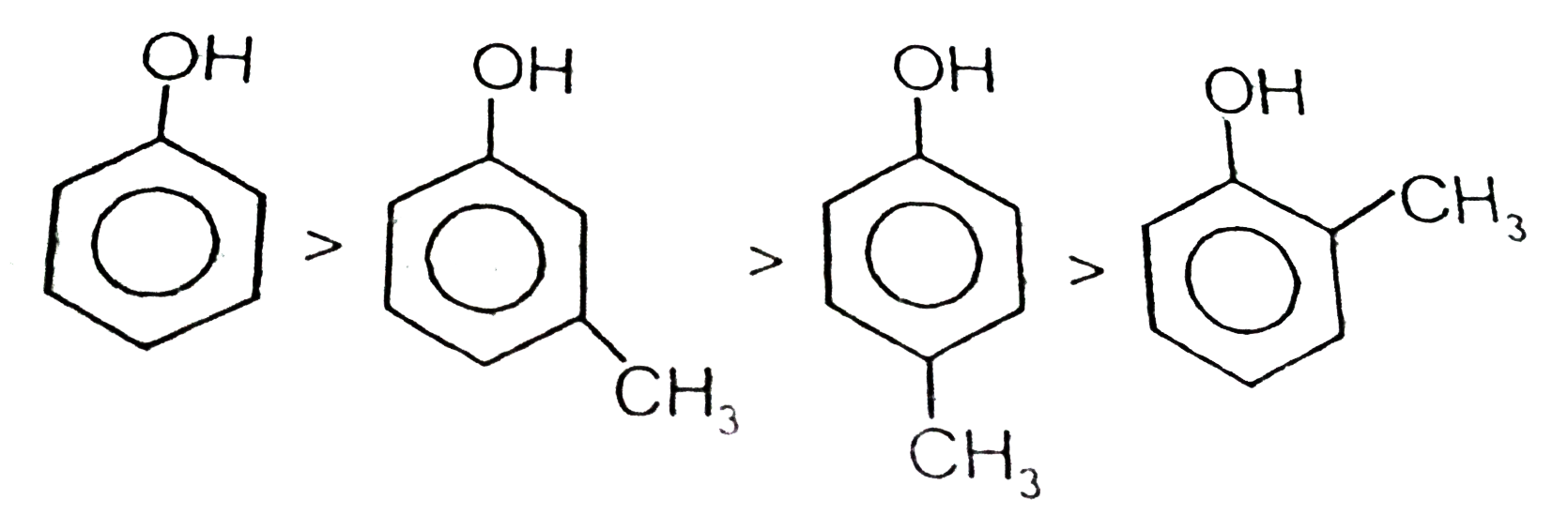
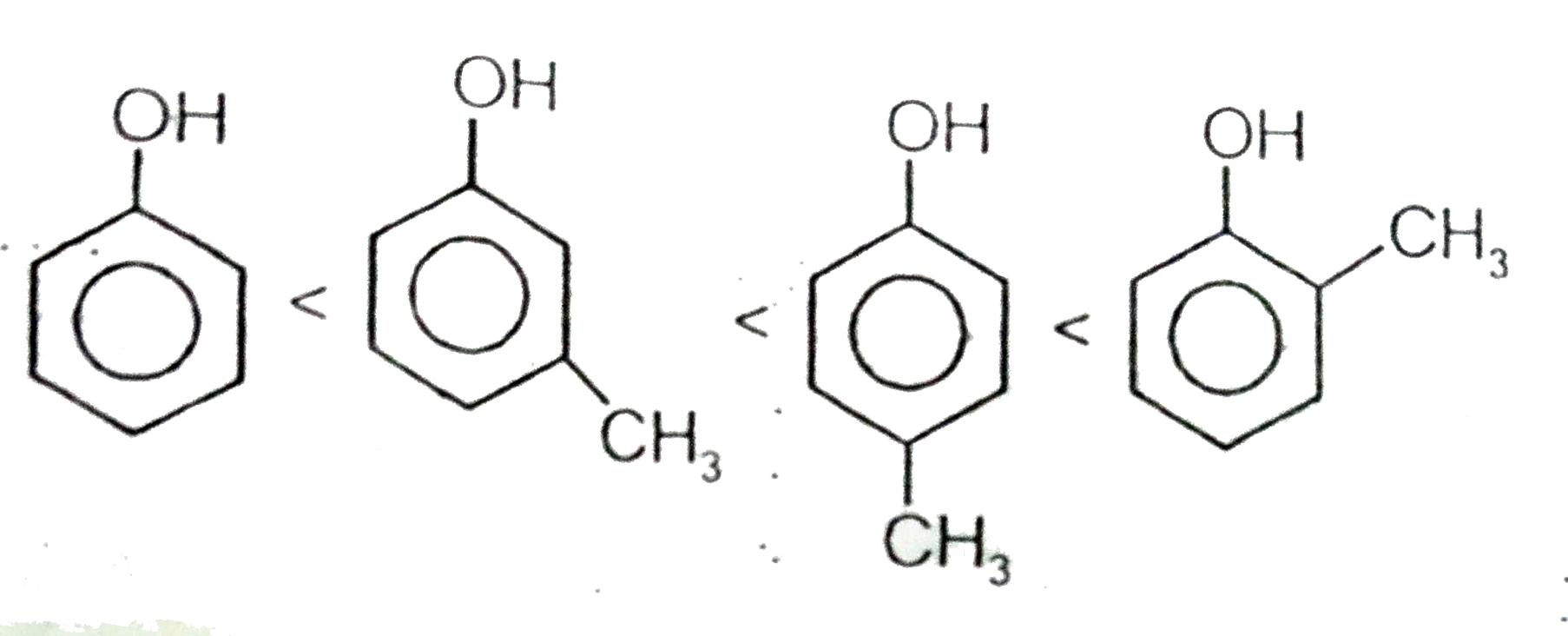
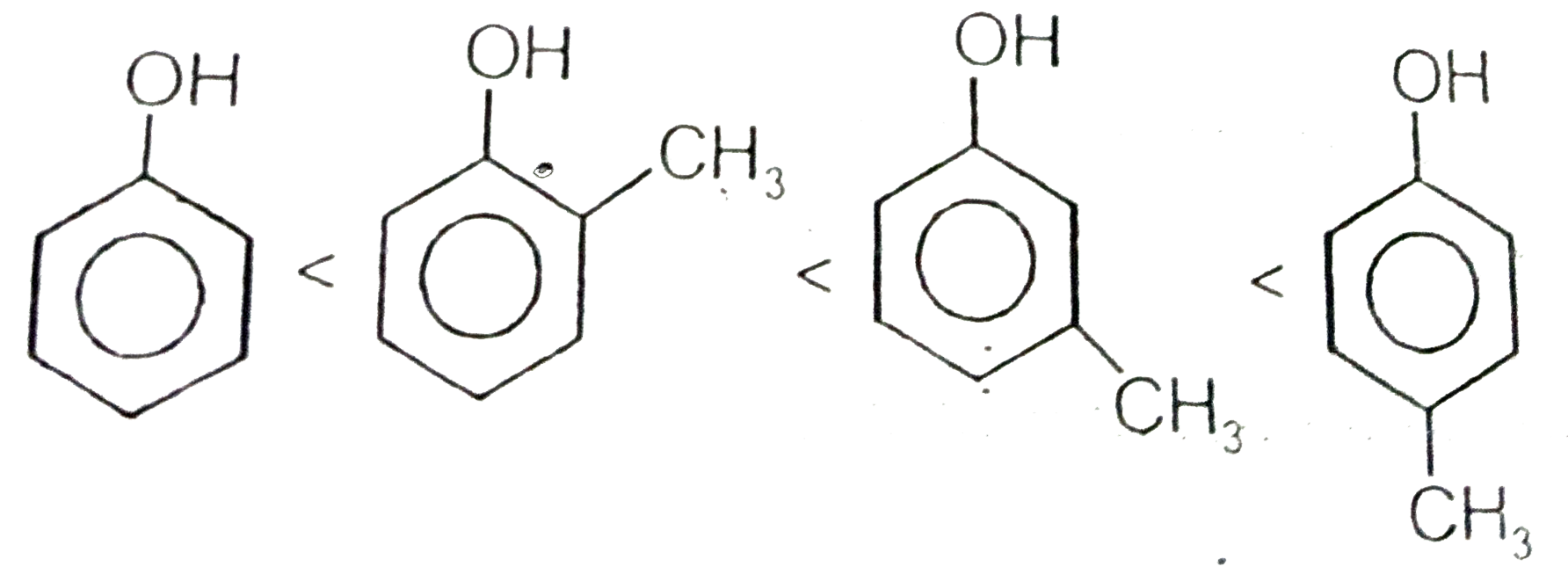
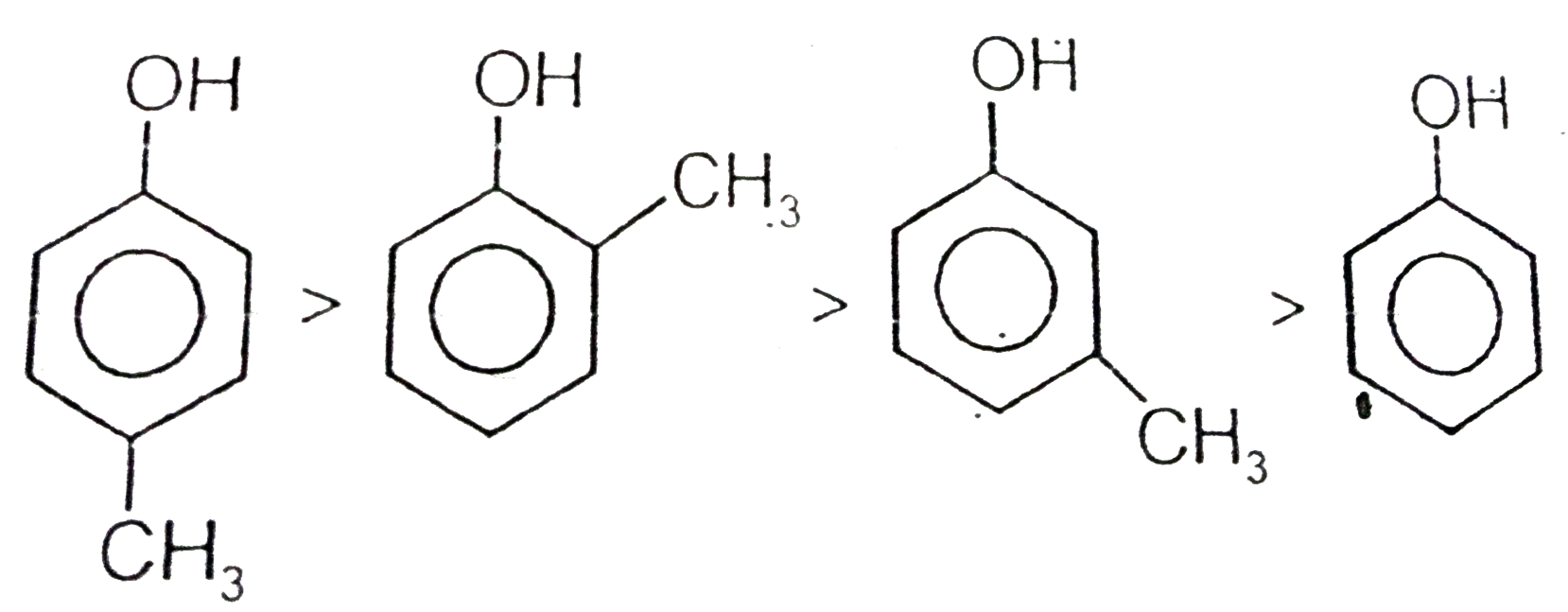
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Correct acidic order is
Text Solution
|
- The set with the correct order of acidity is
Text Solution
|
- The correct acidity order of the following is:
Text Solution
|
- Select the correct order of acidity :
Text Solution
|
- The set with correct order of acidity is :
Text Solution
|
- The correct order of acidity for the following is
Text Solution
|
- For dibasic acid correct order is
Text Solution
|
- The correct increasing order of acidity is:
Text Solution
|
- The correct order of acidity is
Text Solution
|