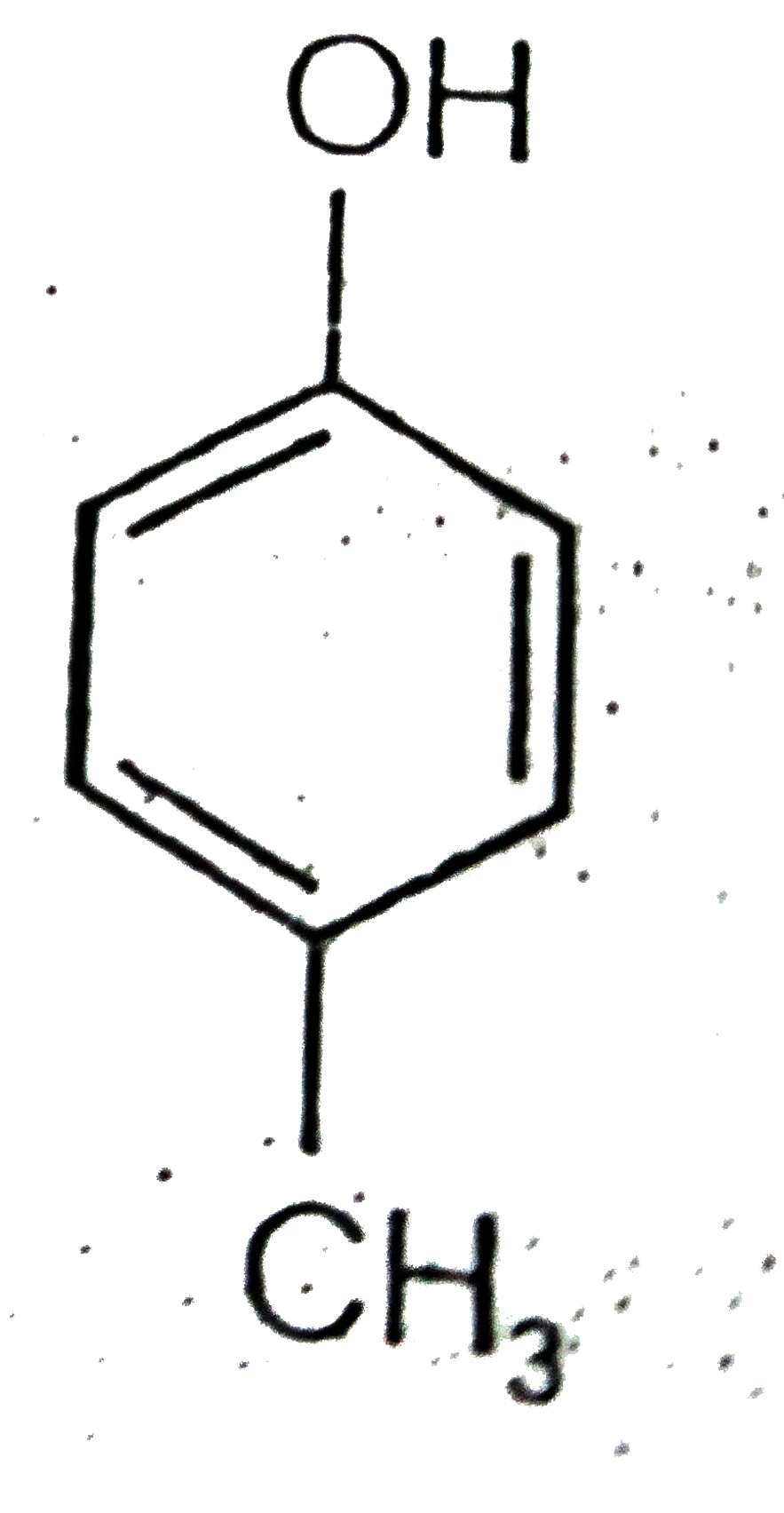
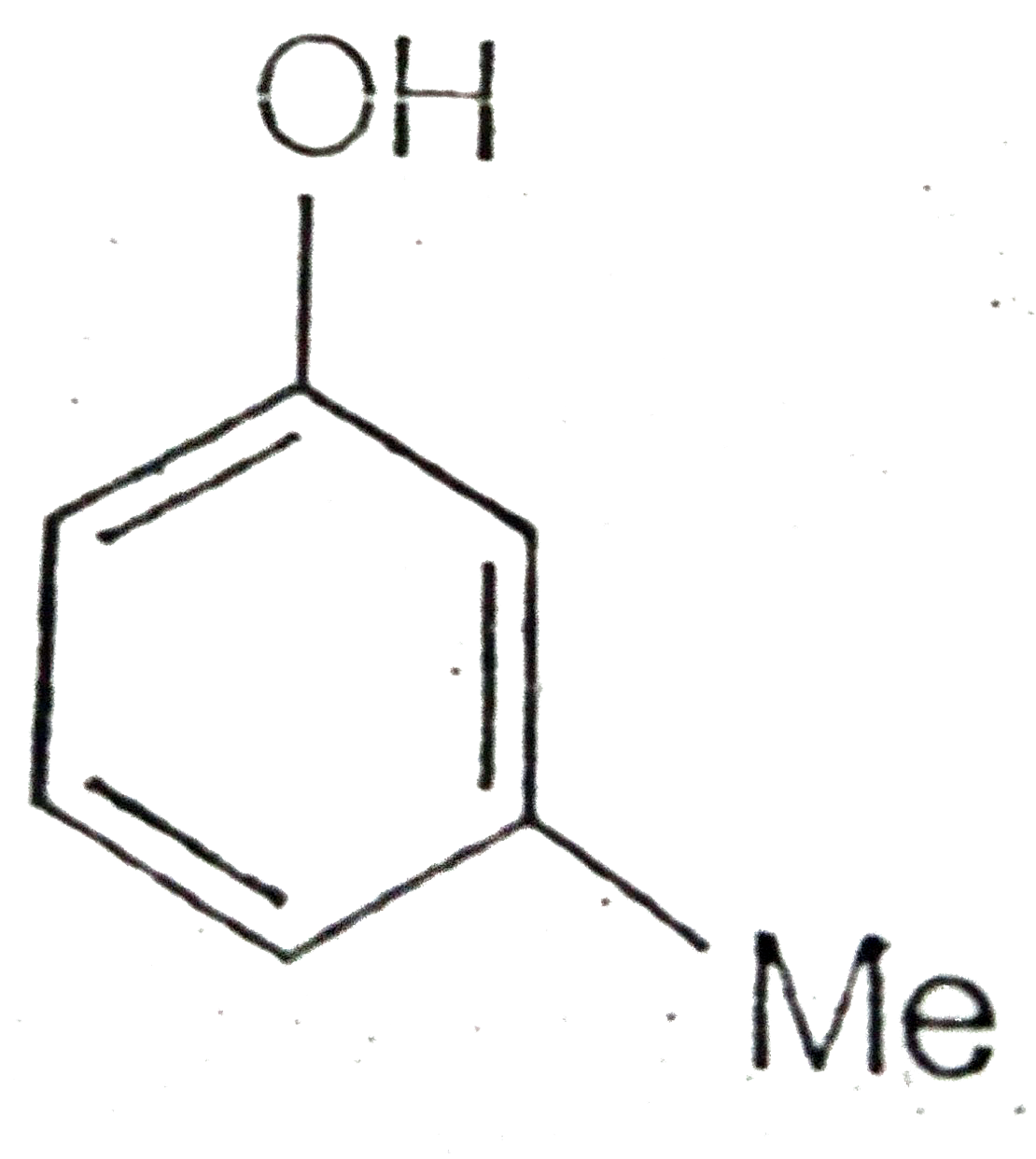
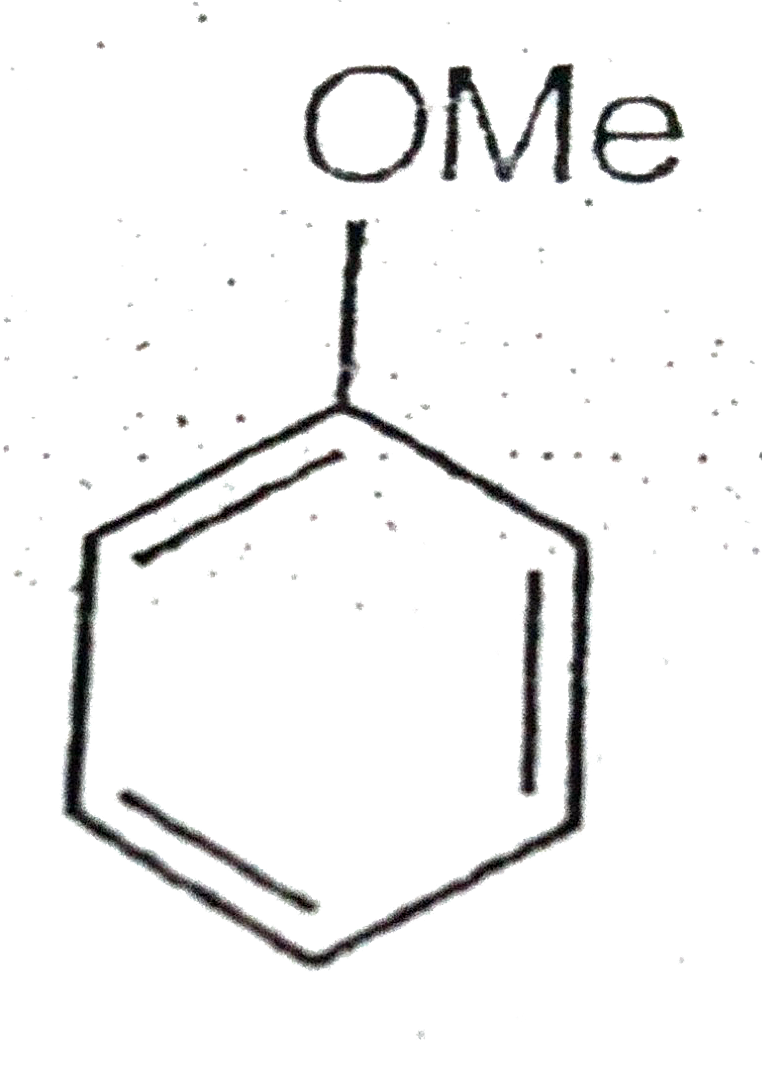
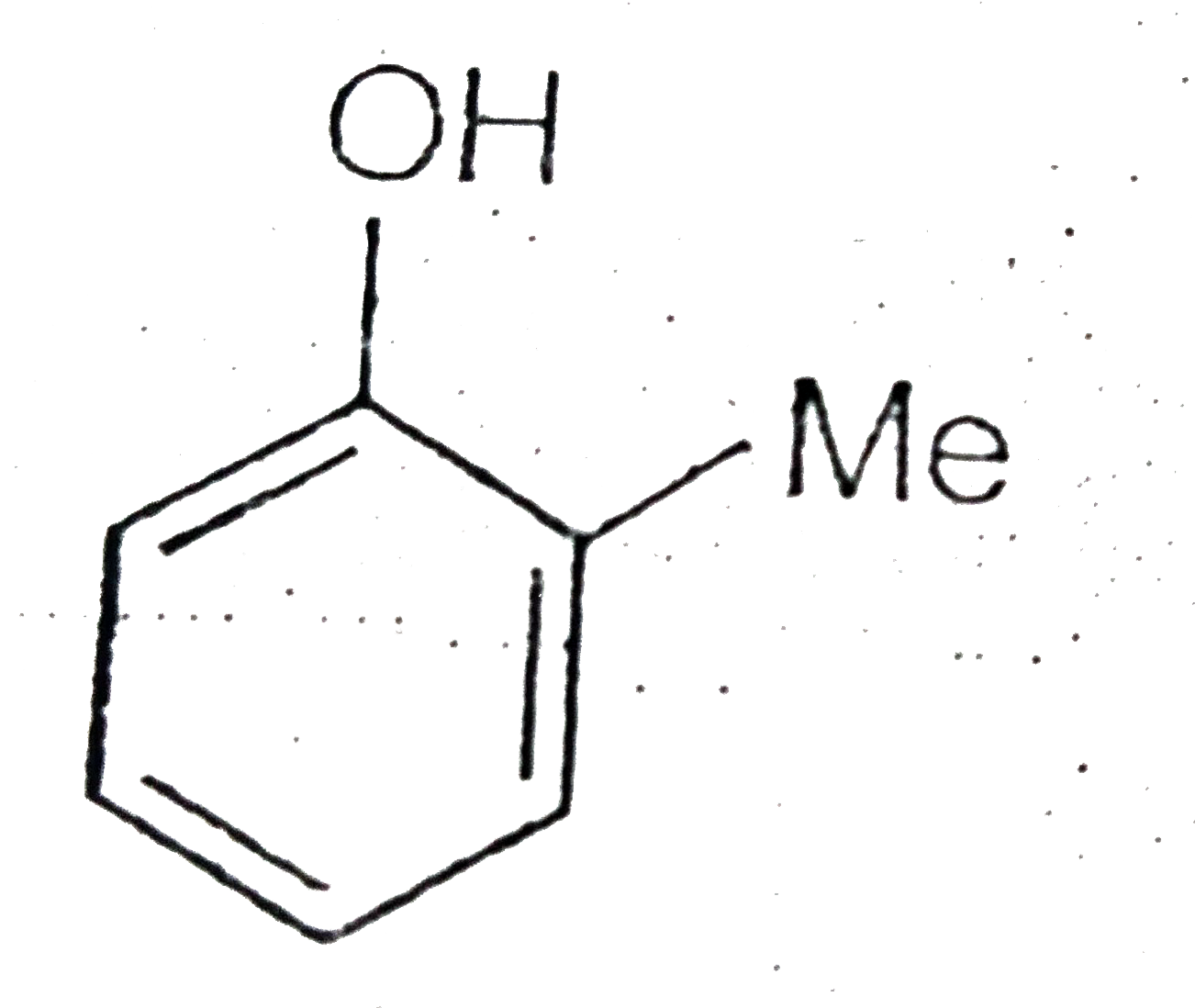
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound (X ) C(7)H(8)O dissolves in NaOH but not in NaHCO(3) ( X)...
Text Solution
|
- An organic compound (A) with molecular formula C(7)H(8)O dissolves in ...
Text Solution
|
- Compound 'P', C(7)H(8)O is insolution in water , dilute when HCI and N...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound A (C(7) H(8) O) is insoluble in water, dilute HCl & aqueous N...
Text Solution
|
- An organic compound (X) with molecular formula C(7)H(8)O is insoluble ...
Text Solution
|