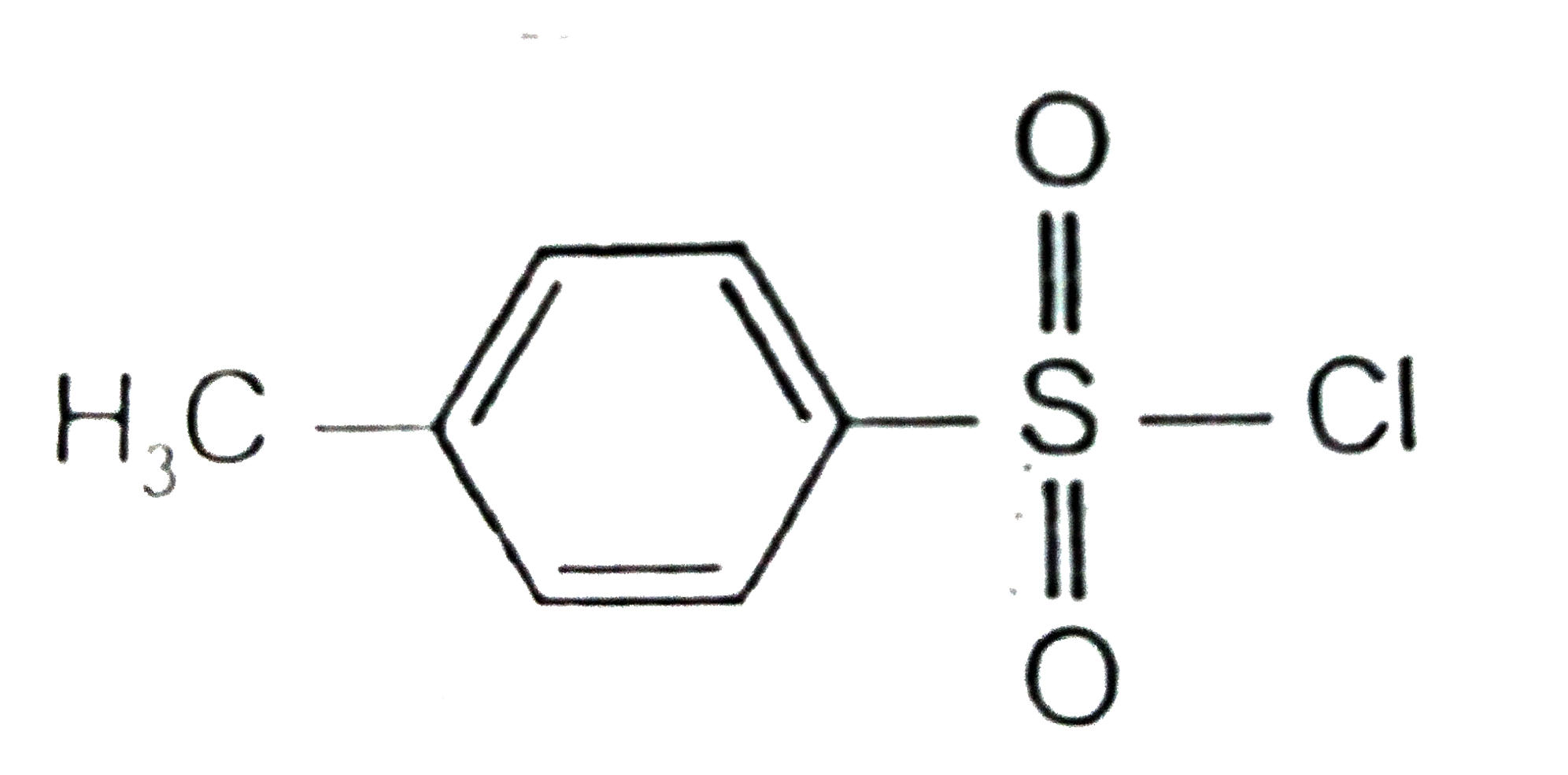
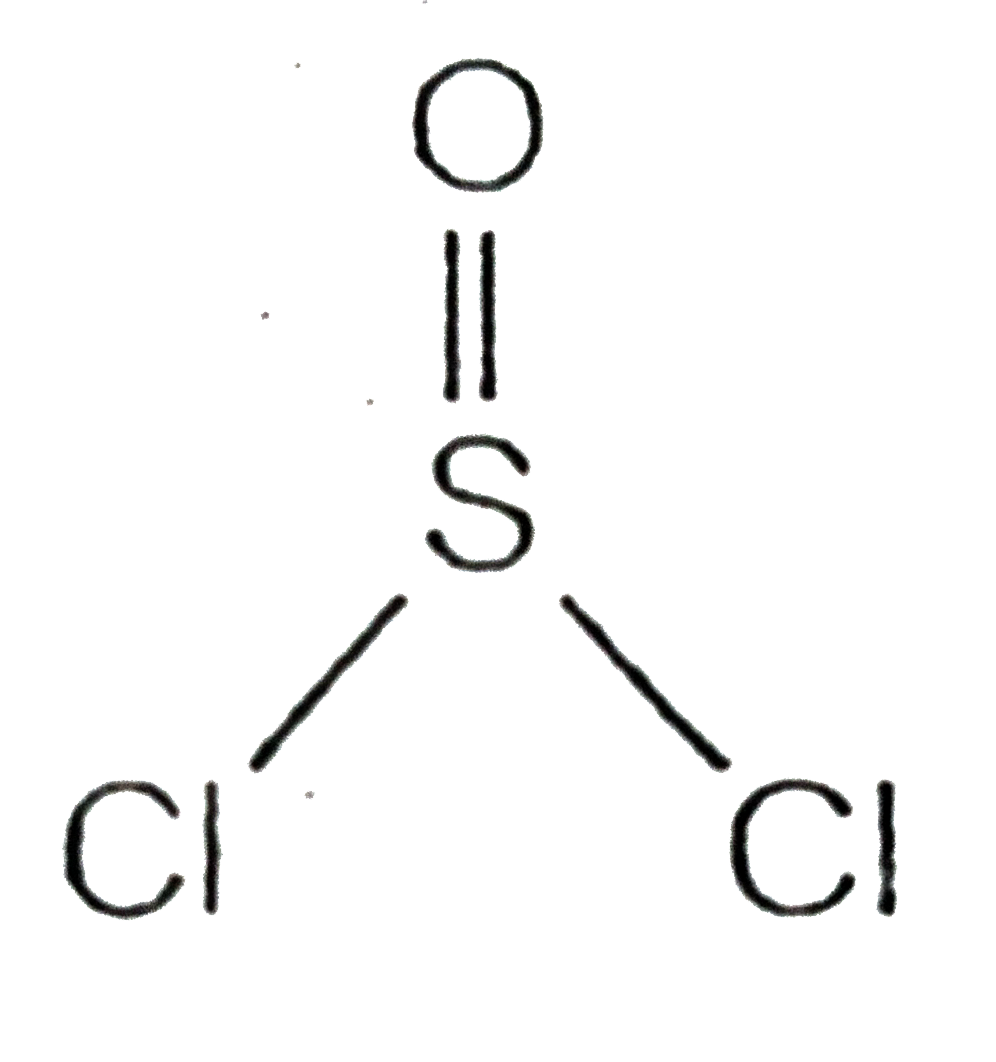
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following convert a primary hydroxyl group into good...
Text Solution
|
- Which is true acidic character of hydroxyl groups of sugars and hydrox...
Text Solution
|
- Nuclephilic substitution reaction is given by those compounds which ha...
Text Solution
|
- Nuclephilic substitution reaction is given by those compounds which ha...
Text Solution
|
- Nuclephilic substitution reaction is given by those compounds which ha...
Text Solution
|
- Which of the following convert a primary hydroxyl group into good leav...
Text Solution
|
- The number of primary and secondary hydroxyl groups in ribose are :
Text Solution
|
- Glucose has primary hydroxyl and. secondary hydroxyl group.
Text Solution
|
- Correct order of leaving group ability for S(N)2 reaction is :
Text Solution
|