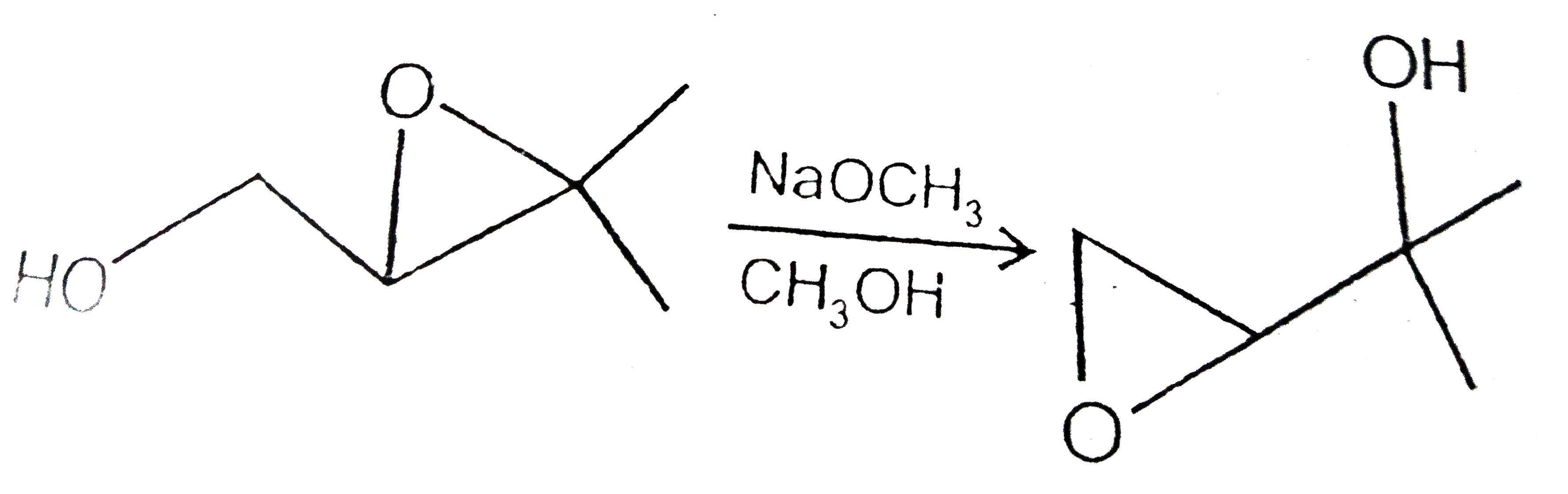
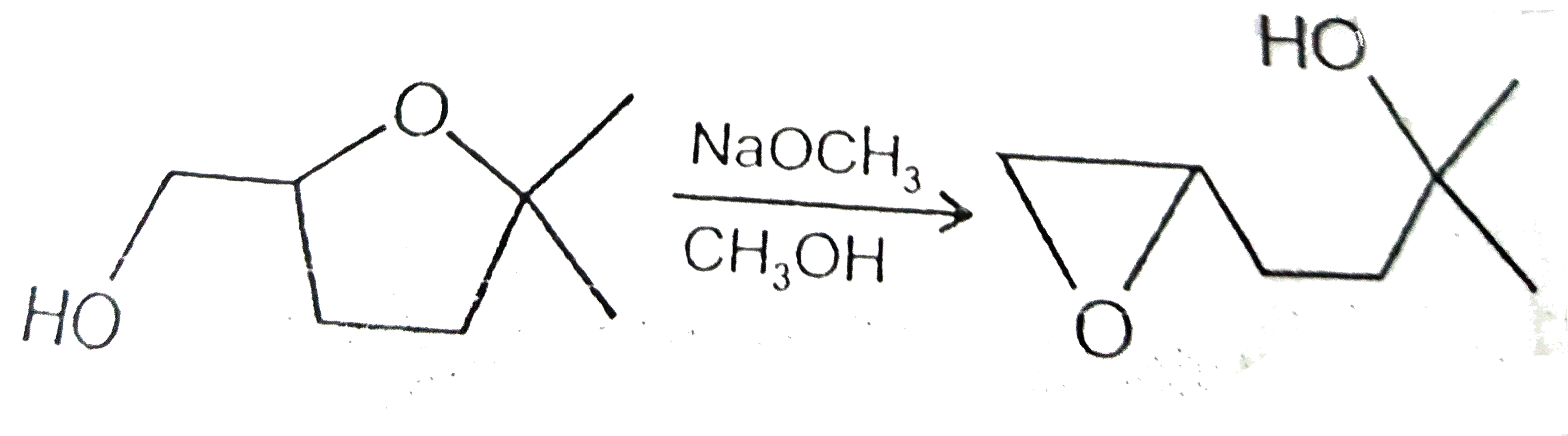
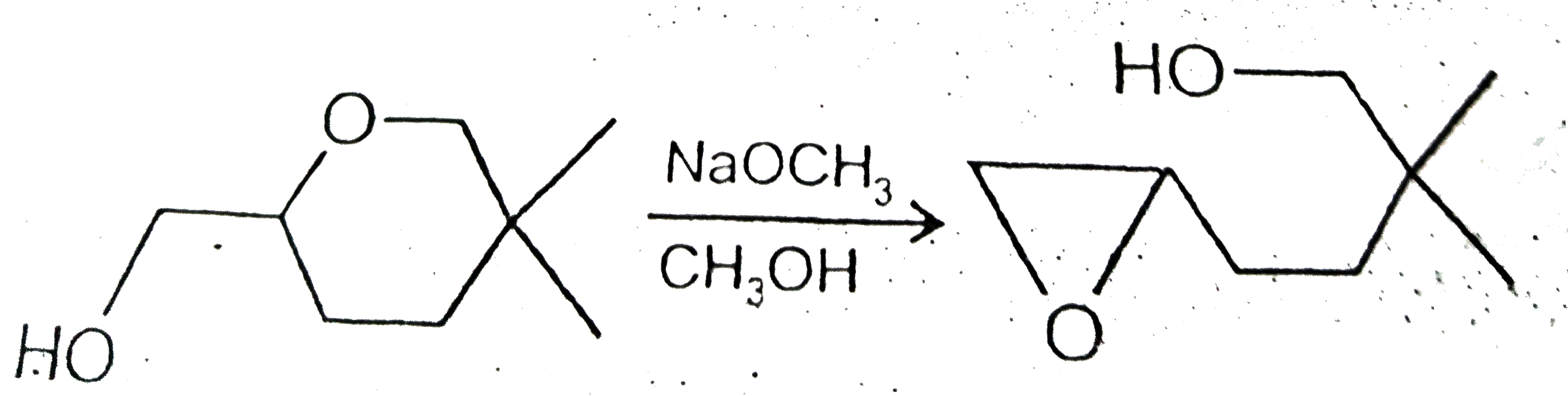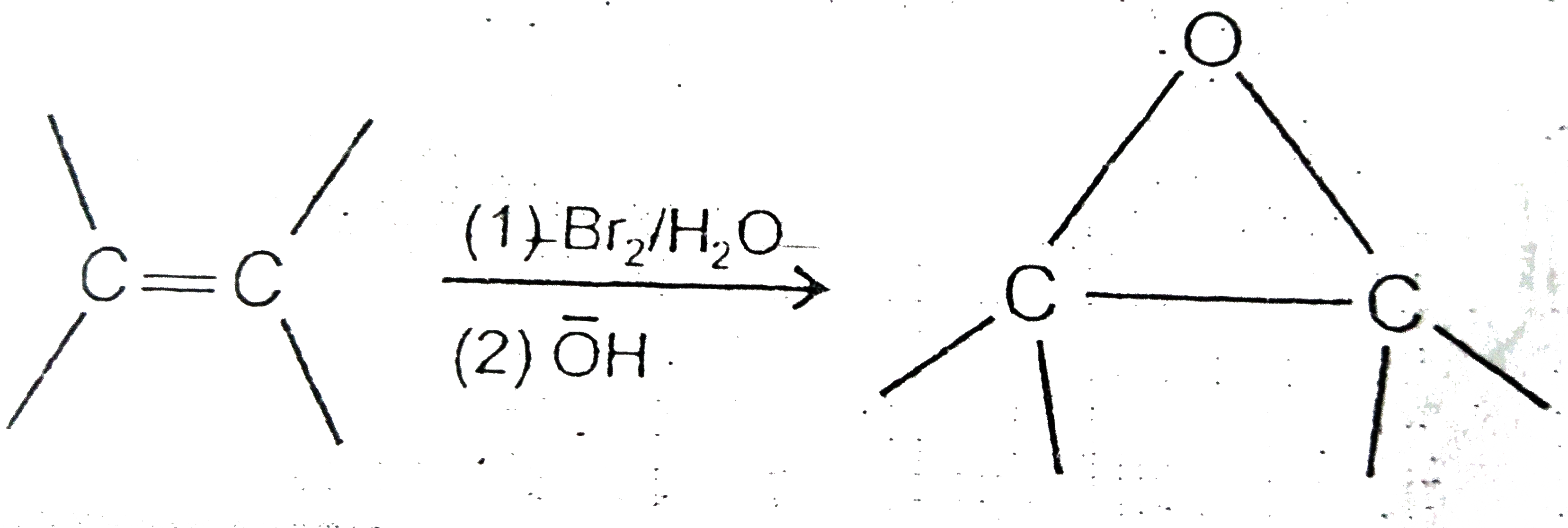A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following reactions will not occur ?
Text Solution
|
- No reaction occurs in which of the following equations?
Text Solution
|
- No reaction occurs in which of the following equations
Text Solution
|
- Which of the following reactions will not occur ?
Text Solution
|
- Which of the following reaction will not occur?
Text Solution
|
- Which of the following reaction do NOT occur ?
Text Solution
|
- Which of the following reaction is not occur in aniline
Text Solution
|
- Polysubstitution occurs for which of the following reactions-
Text Solution
|
- निम्न में से कौन-सी अभिक्रिया नहीं होगी?
Text Solution
|