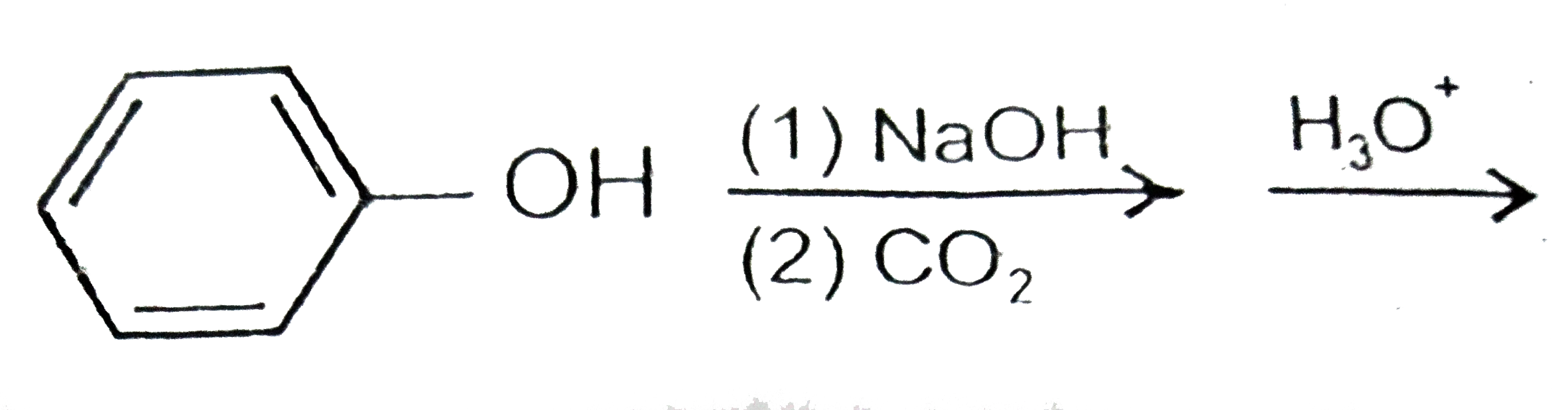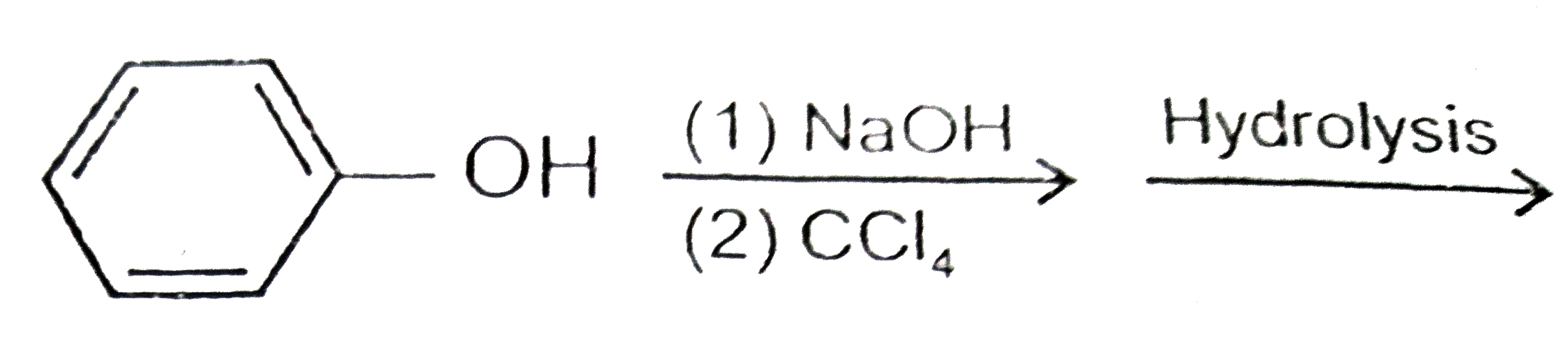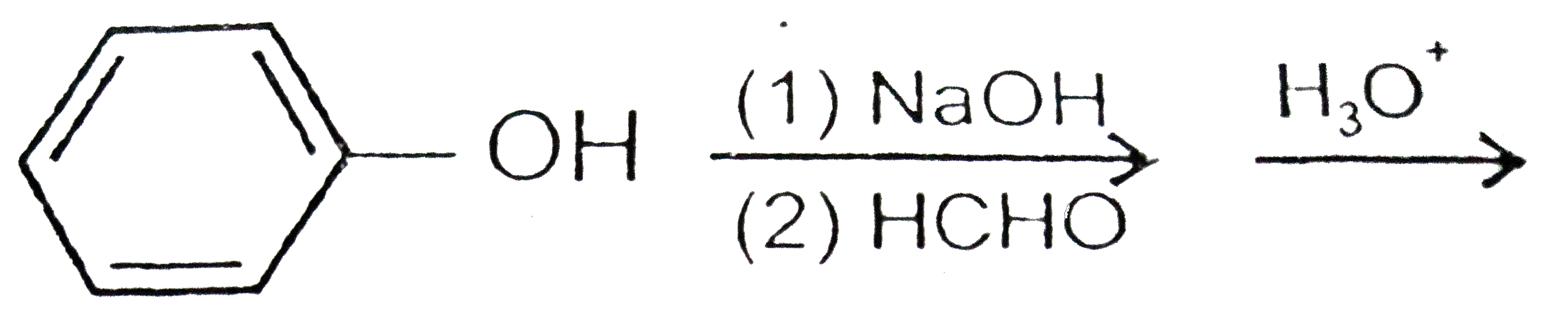A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ortho salicylic acid is frequently used as precursor for the pr...
Text Solution
|
- Salicylic acid can be prepared using Reimer-Tiemann's reaction by trea...
Text Solution
|
- Salicylic acid is prepared from phenol by
Text Solution
|
- Salicylic acid is prepared from phenol by
Text Solution
|
- Phenols can be converted into salicylic acid using
Text Solution
|
- The reagent( s) used in the preparation of aspirin from salicylic acid...
Text Solution
|
- Salicylic acid is prepared from phenol by :
Text Solution
|
- Ortho salicylic acid is frequently used as precursor for the pr...
Text Solution
|
- Which is used to obtain salicylic acid from phenol ?
Text Solution
|