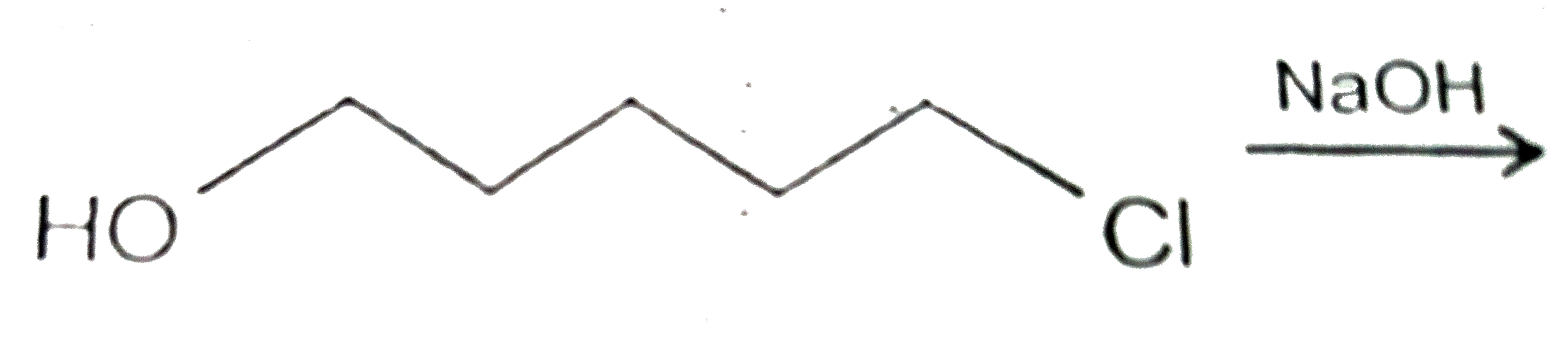
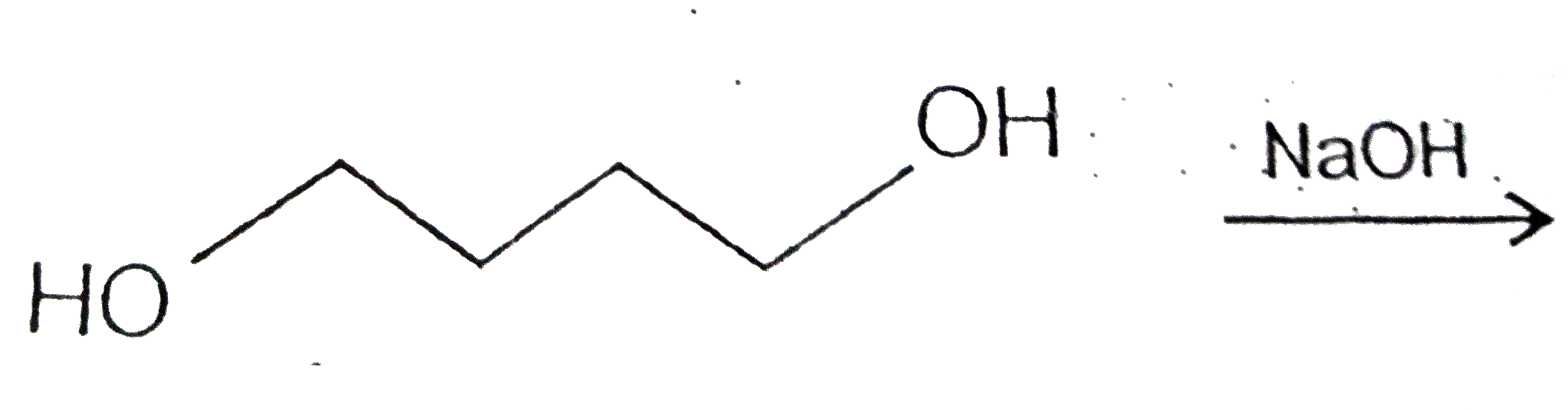
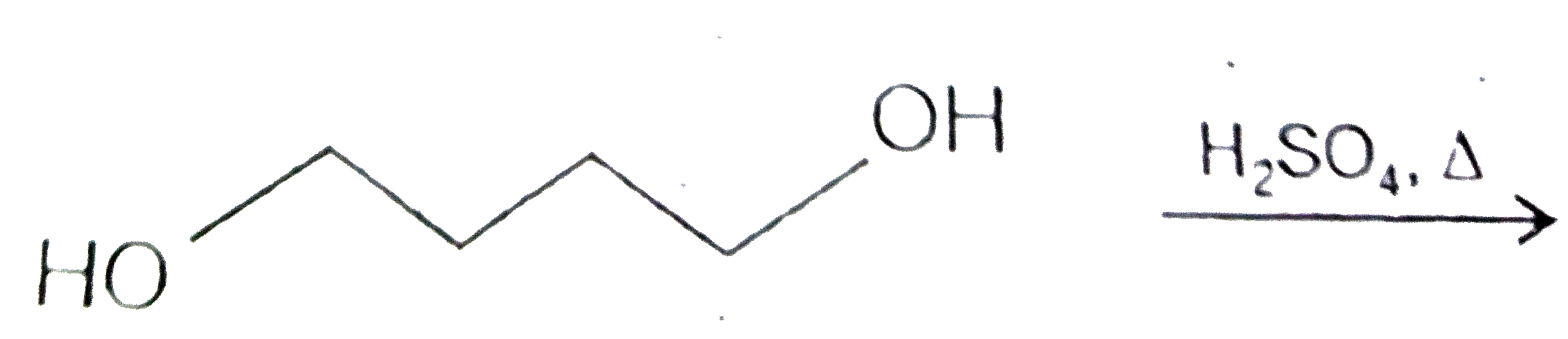
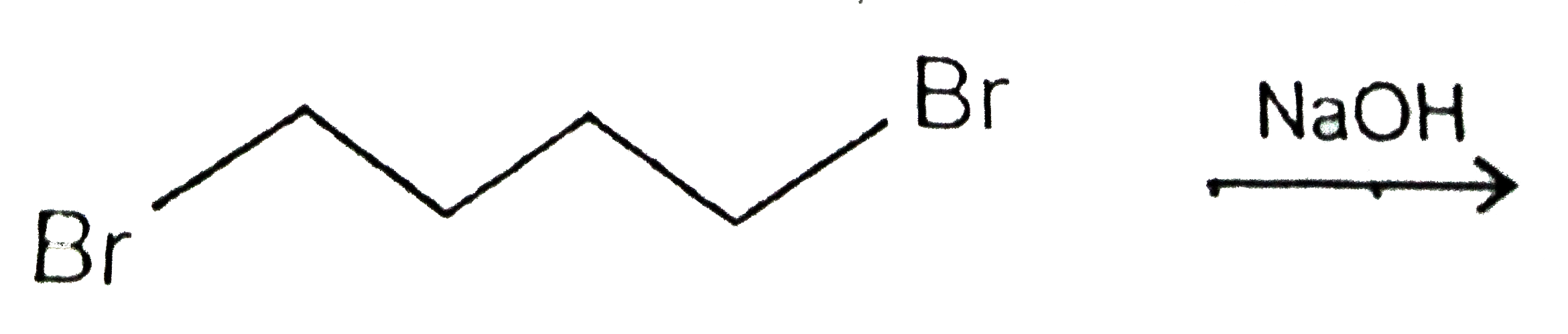
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following reaction can be used to prepare cyclic eth...
Text Solution
|
- Which of the following reactions can be used to prepare lactones (cycl...
Text Solution
|
- Which of the following reactions can be used to prepare acetophenone ?
Text Solution
|
- Which of the reaction can be employed for the preparation of unsymmetr...
Text Solution
|
- Which of the following is a cyclic ether-
Text Solution
|
- Which of the following reaction can be used to prepare cyclic eth...
Text Solution
|
- Which of the following reactions can be used to prepare alkanes?
Text Solution
|
- Which of the following can be used to prepare methyl phenyl ether?
Text Solution
|
- Which of the following reactions can be used to prepare acetophenone?
Text Solution
|