Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the final product , the number of pi electrons involved in aro...
Text Solution
|
- If [sin x]+[(x)/(2 pi)]+[(2x)/(5 pi)]=(9x)/(10 pi), then number of val...
Text Solution
|
- Borazole is aromatic is nature. Nitrigen contributes pi - electrons ...
Text Solution
|
- The number of pi bonds in the final product is
Text Solution
|
- x= number of compound which undergoes Tautomerisation ot from an Aroma...
Text Solution
|
- The number of pi electrons required for a planar cyclic conjugated sys...
Text Solution
|
- In final product the number of pi electrons involved in aromat...
Text Solution
|
- In the final product , the number of pi electrons involved in aromatic...
Text Solution
|
- pi-इलेक्ट्रॉन उपस्थित होने पर वलय तन्त्र ऐरोमैटिक लक्षण दर्शाता है।
Text Solution
|
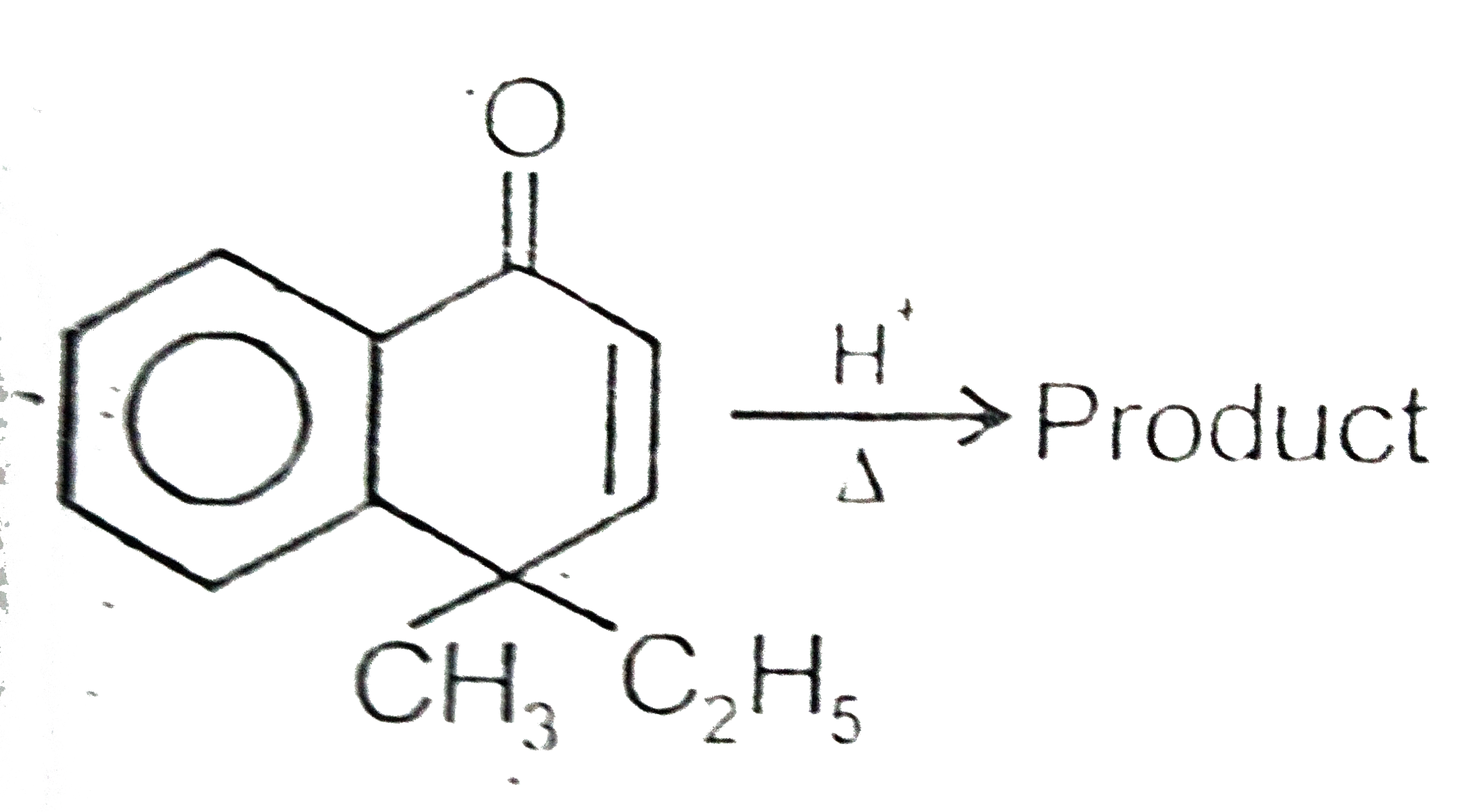 In the final product , the number of `pi` electrons involved in aromaticity is 2x . The value of x is
In the final product , the number of `pi` electrons involved in aromaticity is 2x . The value of x is