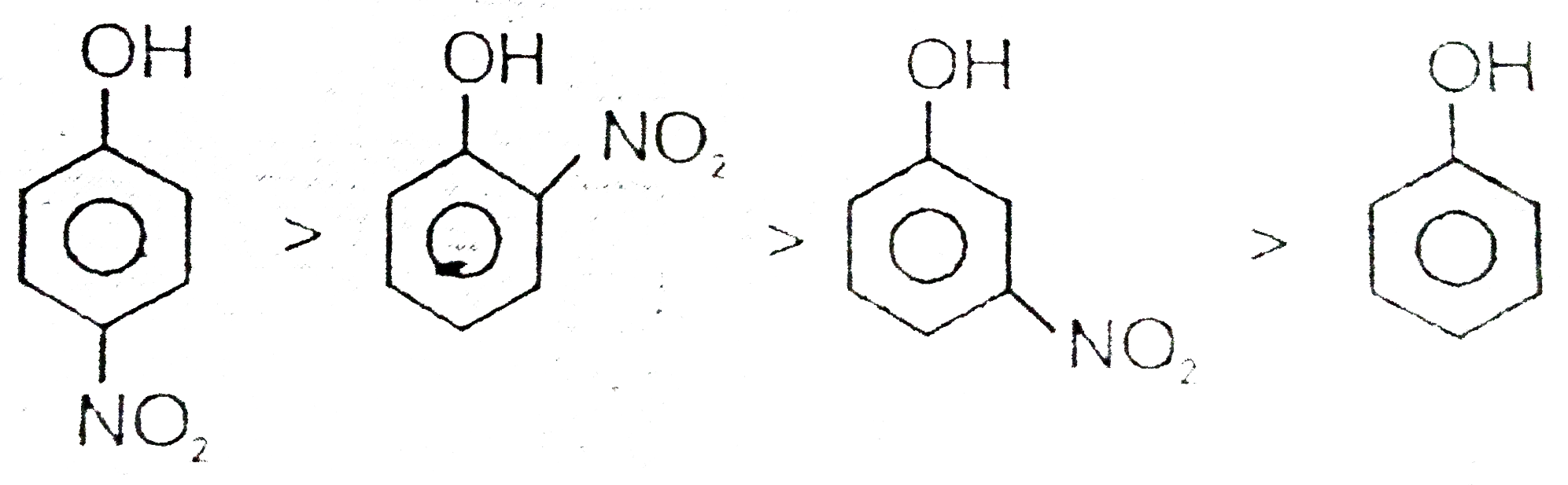
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Why acidic nature of alcohol and phenol increase with electr...
Text Solution
|
- Phenols are more acidic than aliphatic alcohols acidity of phenols can...
Text Solution
|
- (a) Why acidic nature of alcohol and phenol increase with electron wit...
Text Solution
|
- फिनॉल की अम्लीय प्रकृति को प्रदर्शित करने के लिए दो अभिक्रियाएँ दीजिए।...
Text Solution
|
- फिनॉल को कार्बोलिक अम्ल क्यों कहते हैं? इसके अम्लीय स्वभाव की व्याख्या...
Text Solution
|
- Explain the acidic nature of phenols and compare with that of alcohols...
Text Solution
|
- Explain the acidic nature of phenols.
Text Solution
|
- Explain the acidic nature of phenol.
Text Solution
|
- (a) Why acidic nature of alcohol and phenol increases with electron wi...
Text Solution
|