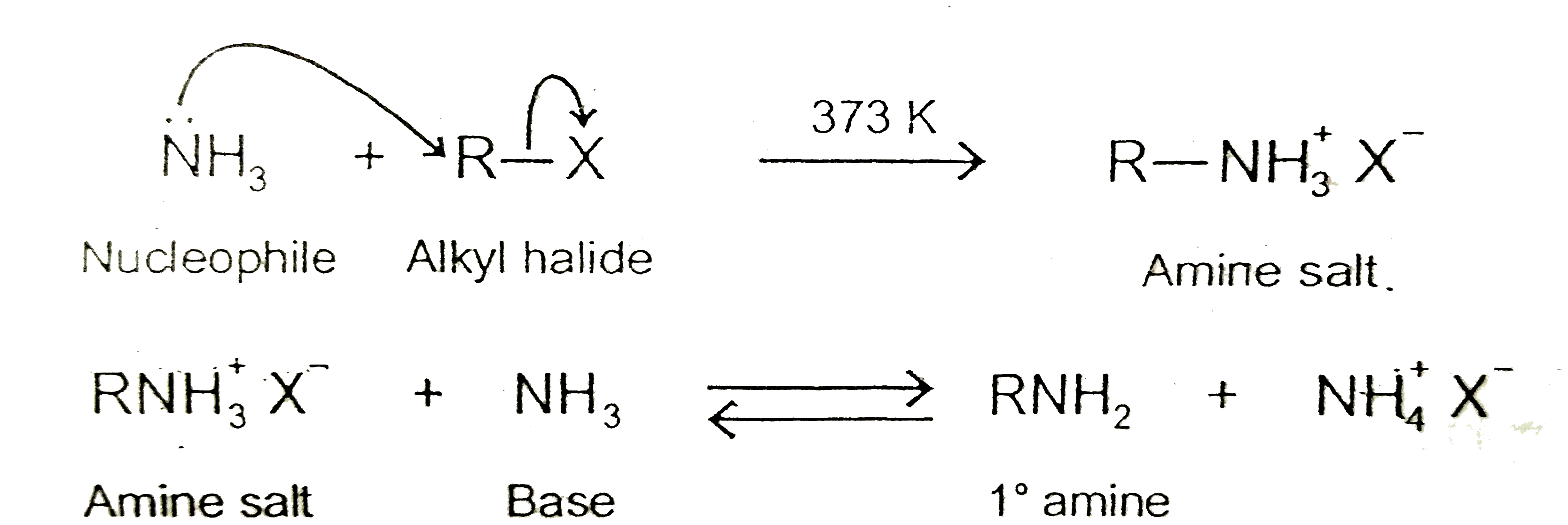Text Solution
Verified by Experts
Topper's Solved these Questions
AMINES
AAKASH INSTITUTE ENGLISH|Exercise ILLUSTRATION|2 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|10 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|20 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-AMINES -Assignment Section -J (Aakash Challengers Questions)
- Why is it difficult to prepare pure amines by ammonolysis of alkyl hal...
Text Solution
|
- Find intermediates and final product in the given sequence of reaction...
Text Solution
|
- The major product obtained in the reaction is
Text Solution
|
- What is the molality of acetic acid solution containing 6 g of acetic ...
Text Solution
|
- Imidazole is a building block of the essential amino acid histidine. W...
Text Solution
|
- Following is an outline for the synthesis of diazepam. A commonly used...
Text Solution
|
- Following is an outline for the synthesis of diazepam. A commonly used...
Text Solution
|
- What is the overall structure of diazepam?
Text Solution
|
- At 1000 K, the value of Kp for the reaction : A(g)+2B(g)⇌3C(g)+D(g) ...
Text Solution
|
- 80% of a first order reaction was completed in 70 min. What is the hal...
Text Solution
|