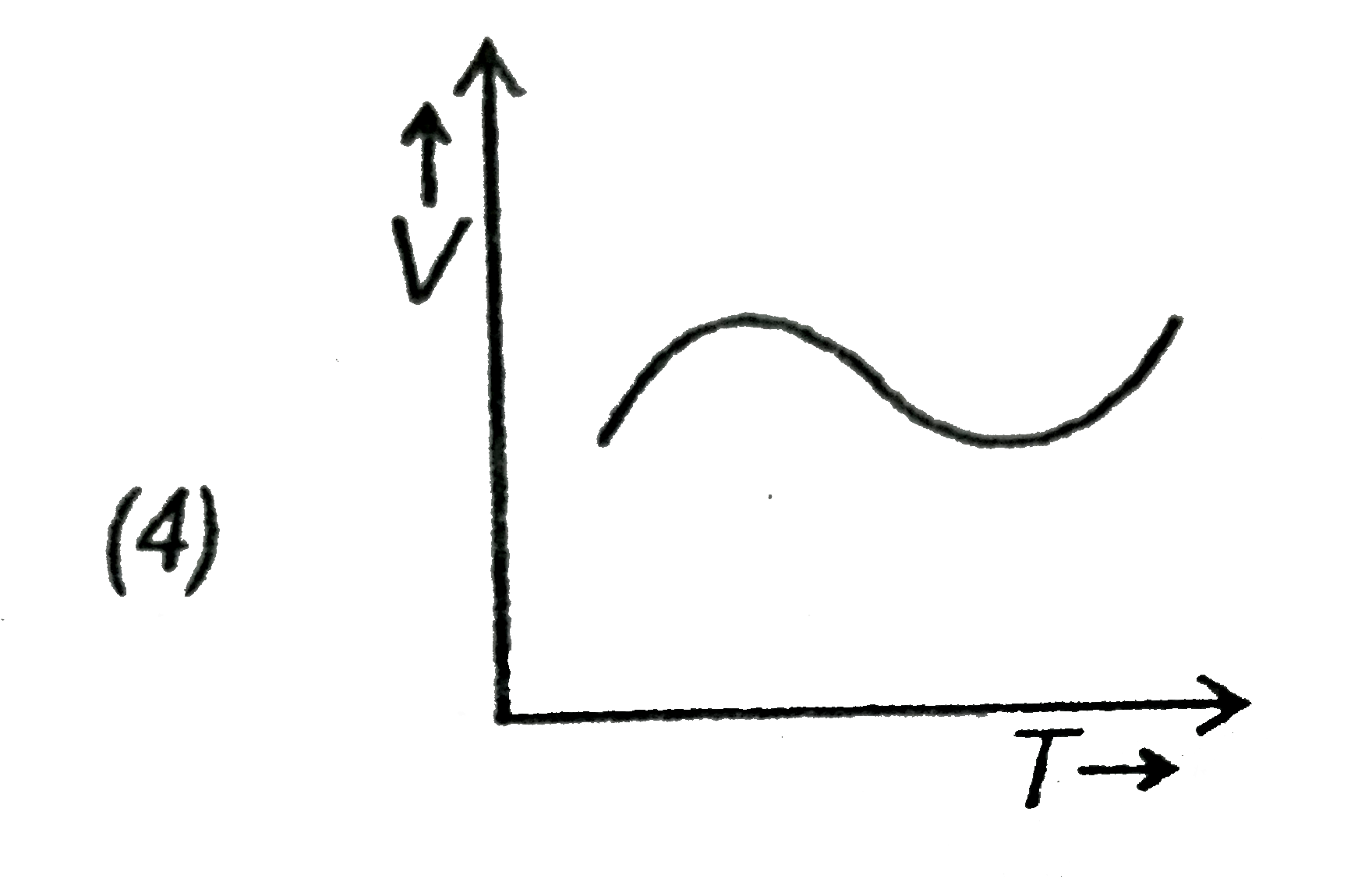A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Try Youself|13 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-A) Objective Type Questions (one option is correct)|50 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-ASSIGNMENT (SECTION -D) (Assertion - Reason Type Questions)
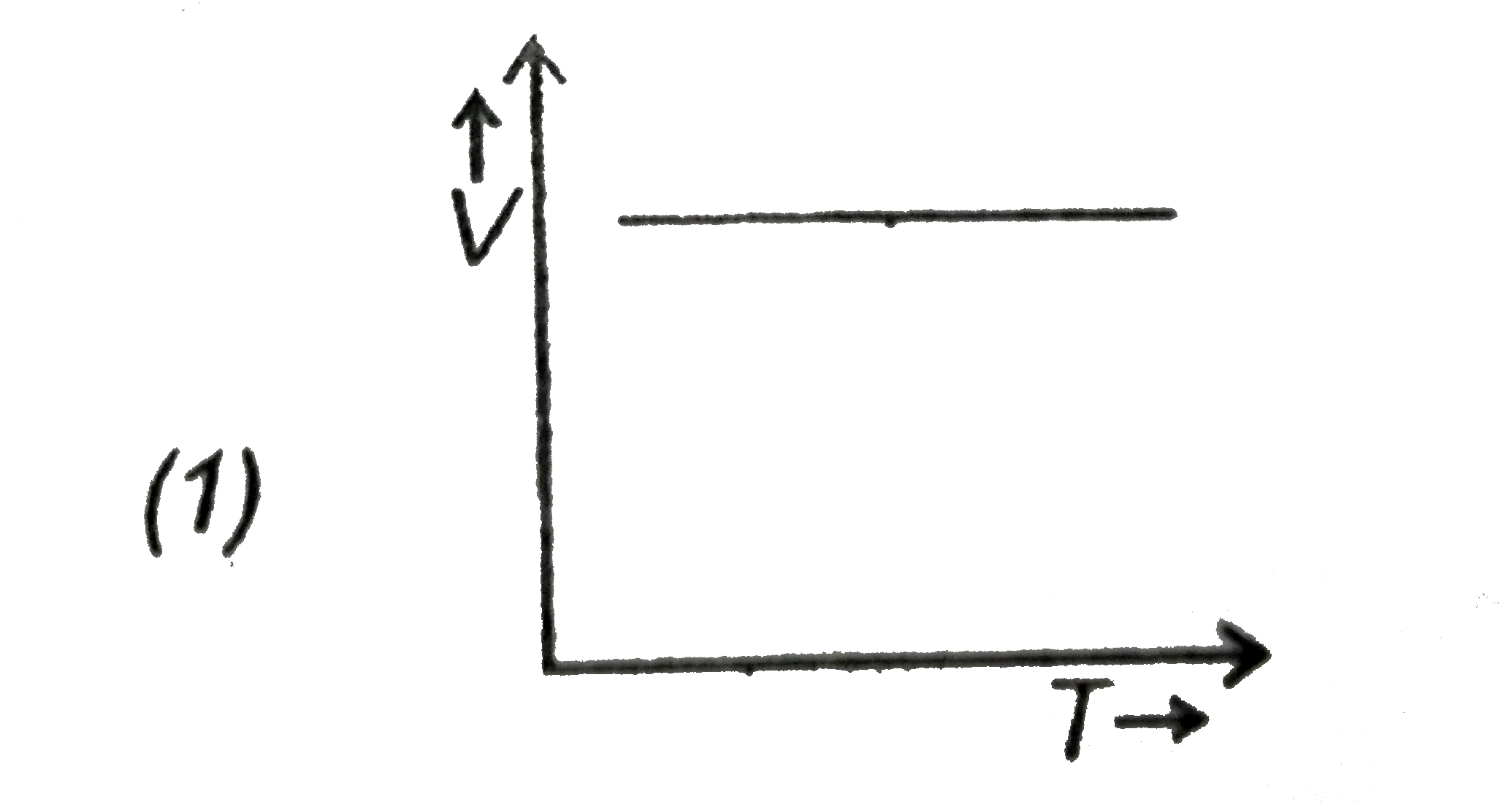
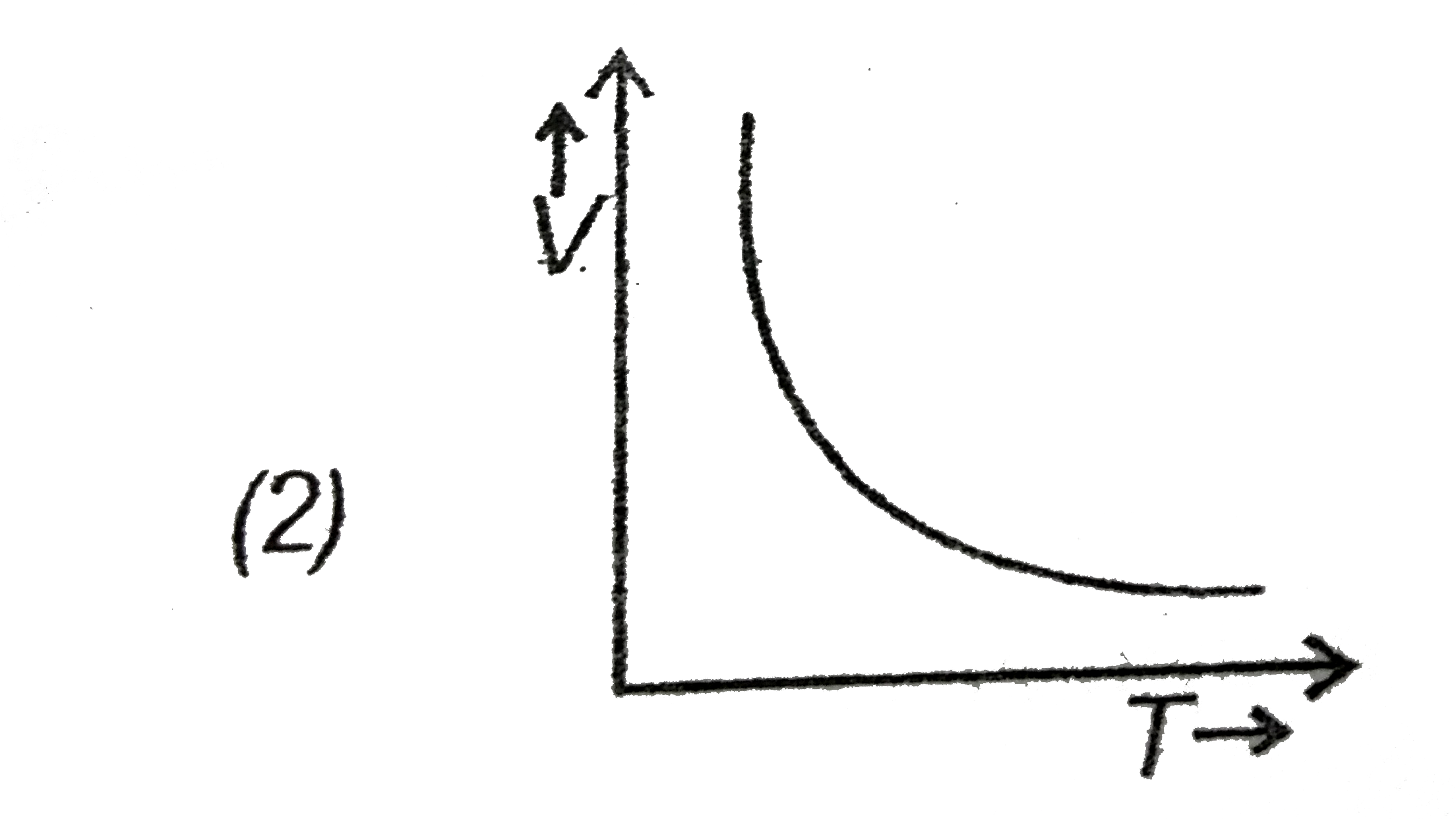
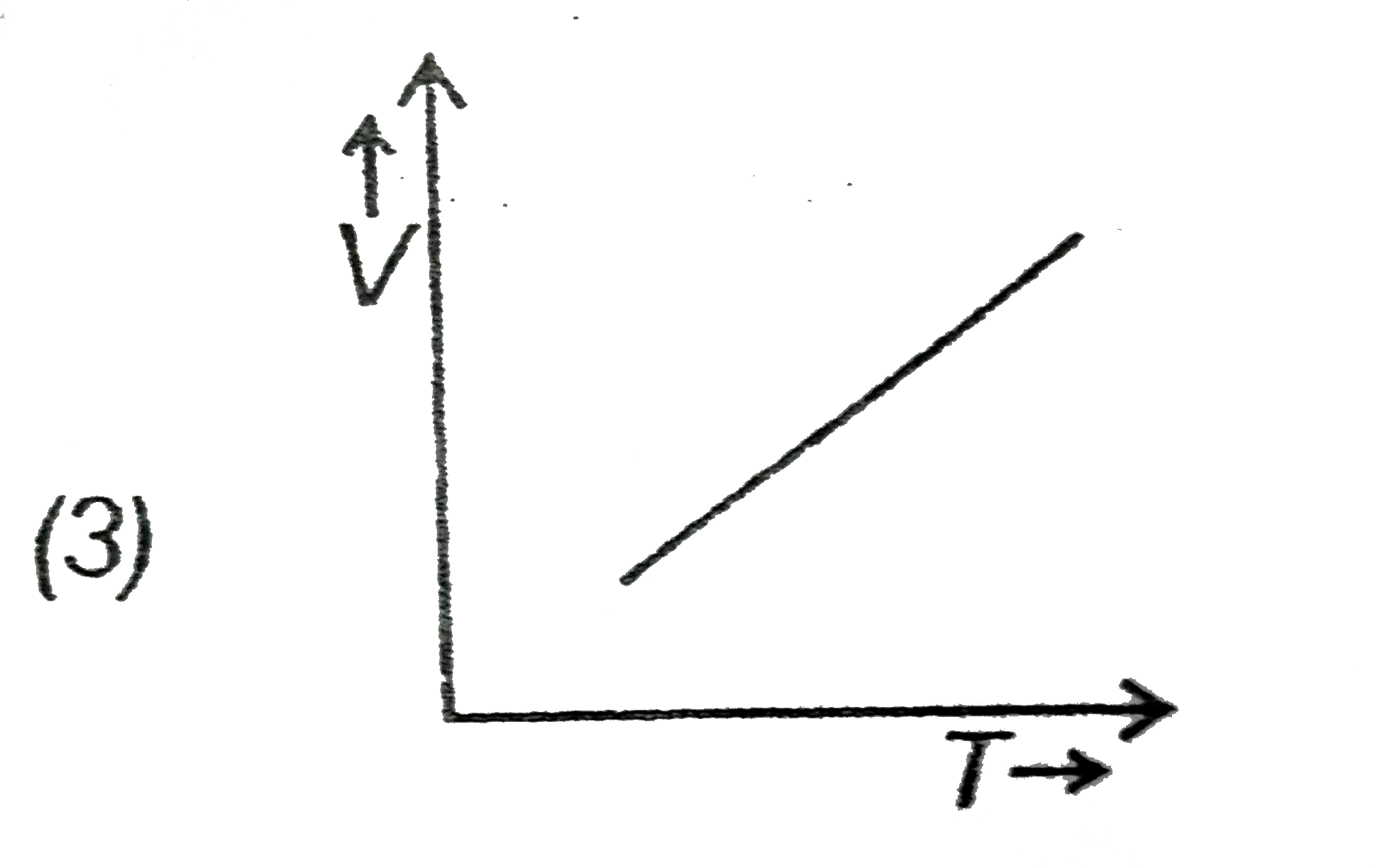
- Which of the following is a V-T curve for isobaric process?
Text Solution
|
- A : Work done by a gas in isothermal expension is more than the work d...
Text Solution
|
- A : Efficiency of heat engine can neve be 100%. R : Second law of th...
Text Solution
|
- A : Heat absorbed in a cyclic process is zero. R : Work done in a cy...
Text Solution
|
- A : Coefficient of performanceof a refreigerator is always greater tha...
Text Solution
|
- A : Adiabatic expansion causes cooling. R : In adiabatic expansion, ...
Text Solution
|
- A : The specific heat of an ideal gas is zero in an adiabatic process....
Text Solution
|
- A : The change in intermal energy does not depend on the path of proce...
Text Solution
|
- A : Heat supplied to a gaseous system in an isothermal process is used...
Text Solution
|
- Assertion: Thermodynamics process in nature are irreversible. Reason...
Text Solution
|
- A : During a cyclic process work done by the system is zero. R : Hea...
Text Solution
|