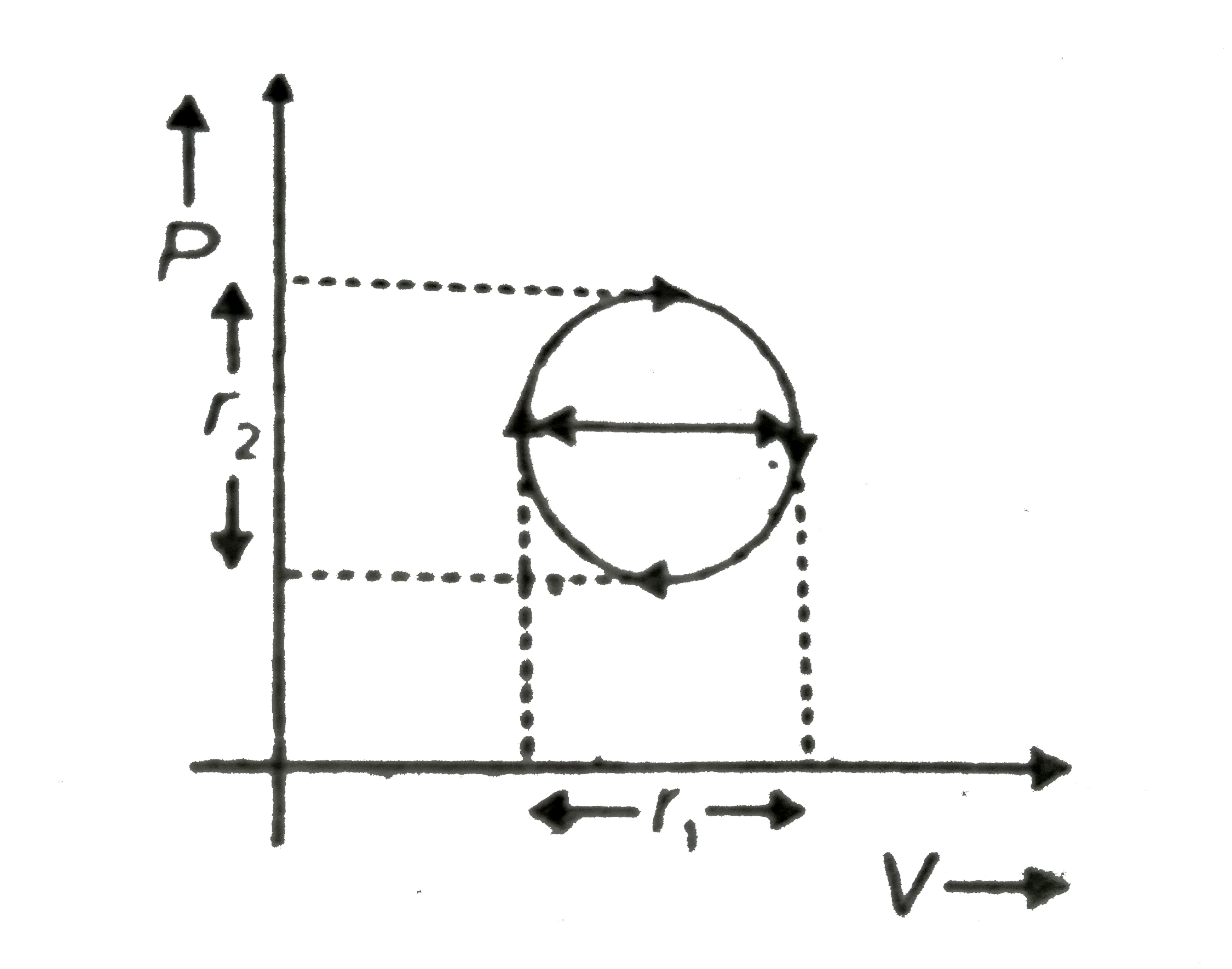
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-C) Objective Type Questions (More than one option are correct)|11 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-D) Linked comprehension Type questions|3 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-A) Objective Type Questions (one option is correct)|50 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-Assignment (Section-B) Objective Type Questions (one option is correct)
- A sample contains N moles of a diatomic gas at temperature T. Molecule...
Text Solution
|
- A sample of gas follows process AB, then which of the following is inc...
Text Solution
|
- A sample of gas is going through cyclic process, work done by gas is
Text Solution
|
- A movable piston separates a sample of gas from vacuum. In this state,...
Text Solution
|
- A container is closed by a movable piston. We supply heat to gas insid...
Text Solution
|
- For a process, relation between temperature and volume is TV^(3)=const...
Text Solution
|
- In a thermodynamic process on an ideal diatomic gas, work done by the ...
Text Solution
|
- An open container is placed in atmosphere. During a time interval, tem...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Total kinetic energy of molecules of a gas sample varies K.E.=(7)/(4)n...
Text Solution
|
- A sample of diatomic gas is heated at constant pressure. If an amount ...
Text Solution
|
- P-V diagram of a diatomic gas is a straight line passing through origi...
Text Solution
|
- A cyclic process contains four steps AB,BC,CD and DA. Heat involved in...
Text Solution
|
- A monatomic ideal gas is heated at constant volume until its pressure ...
Text Solution
|
- A sample of gas goes through a process shown in graph. Arrange the vol...
Text Solution
|