Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-H) Multiple True-False Type Question|2 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-I) Subjective Type|5 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-F) Matrix-Match type Question|5 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
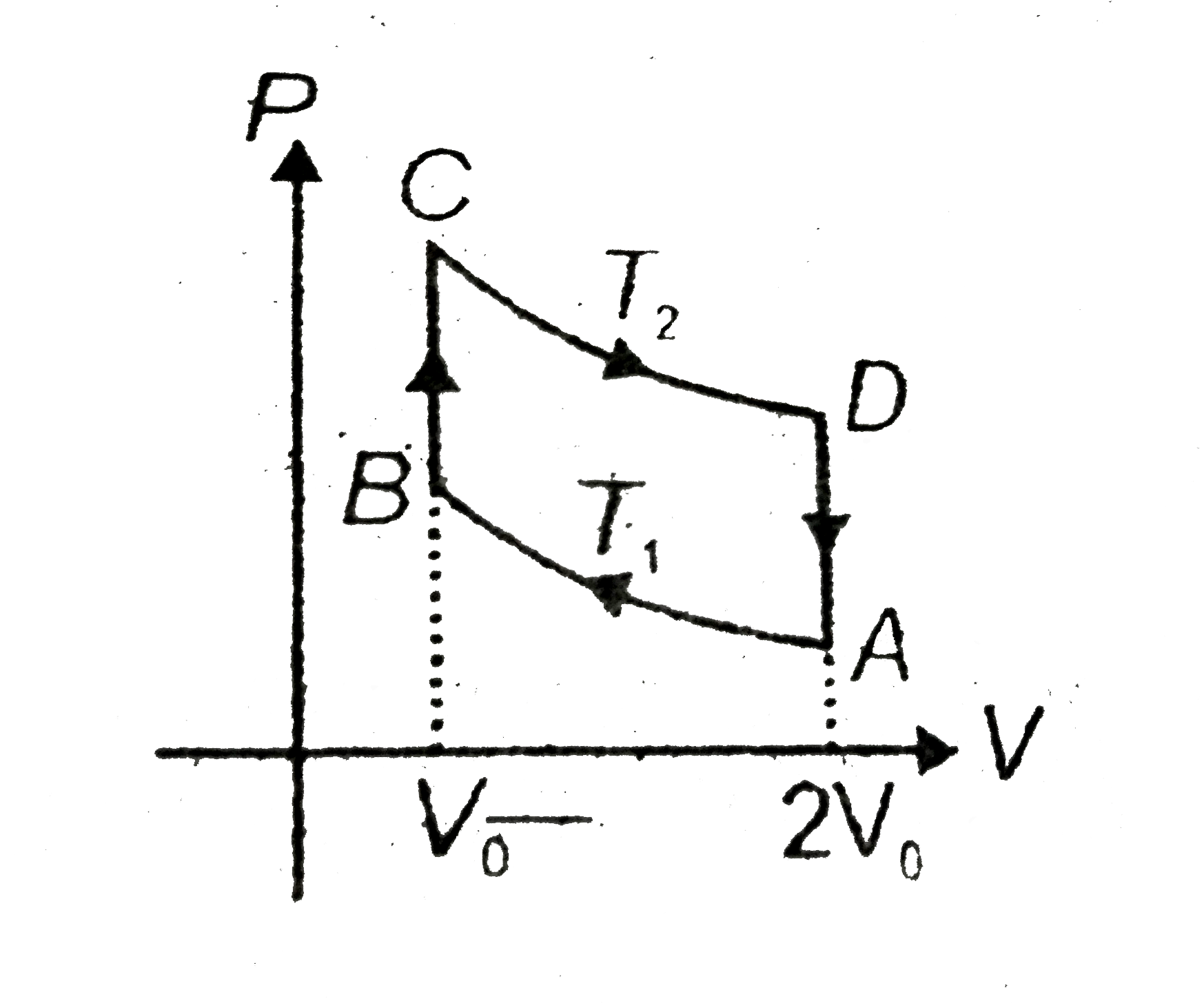 .
.