Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Aakash Challengers Questions|6 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise TRY YOURSELF|12 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-H) Multiple True-False Type Question|2 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-Assignment (Section-I) Subjective Type
- Two moles of an ideal gas are contained in a vertical cylinder with a ...
Text Solution
|
- An ideal monoatomic gas goes through a cyclic process. Ratio of maximu...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes the process T=T(0)+4V, w...
Text Solution
|
- Two moles of a certain gas at a temperature T0=300K were cooled isocho...
Text Solution
|
- The volume of one mole of an ideal gas with the adiabatic exponent gam...
Text Solution
|
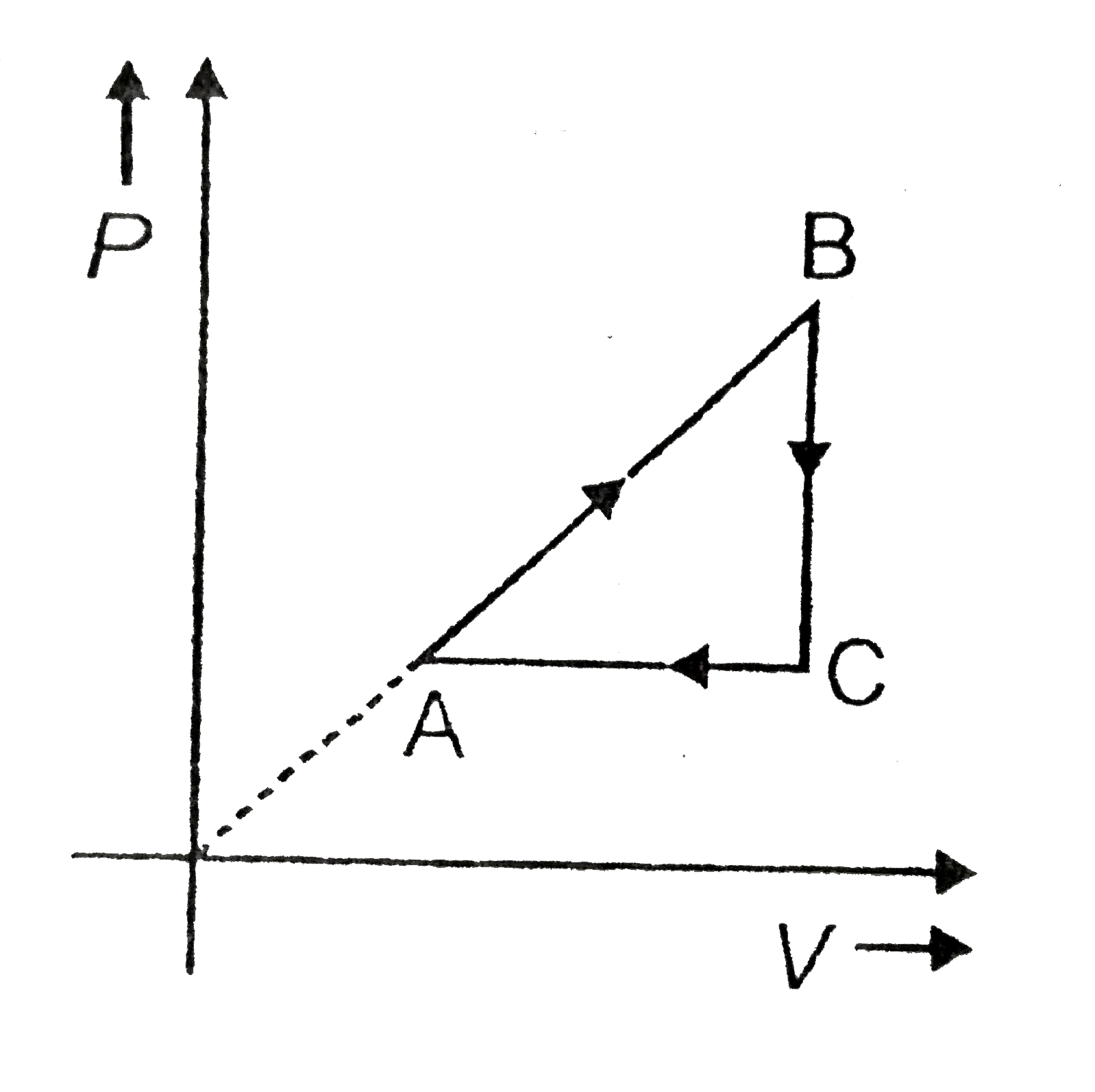 .
.