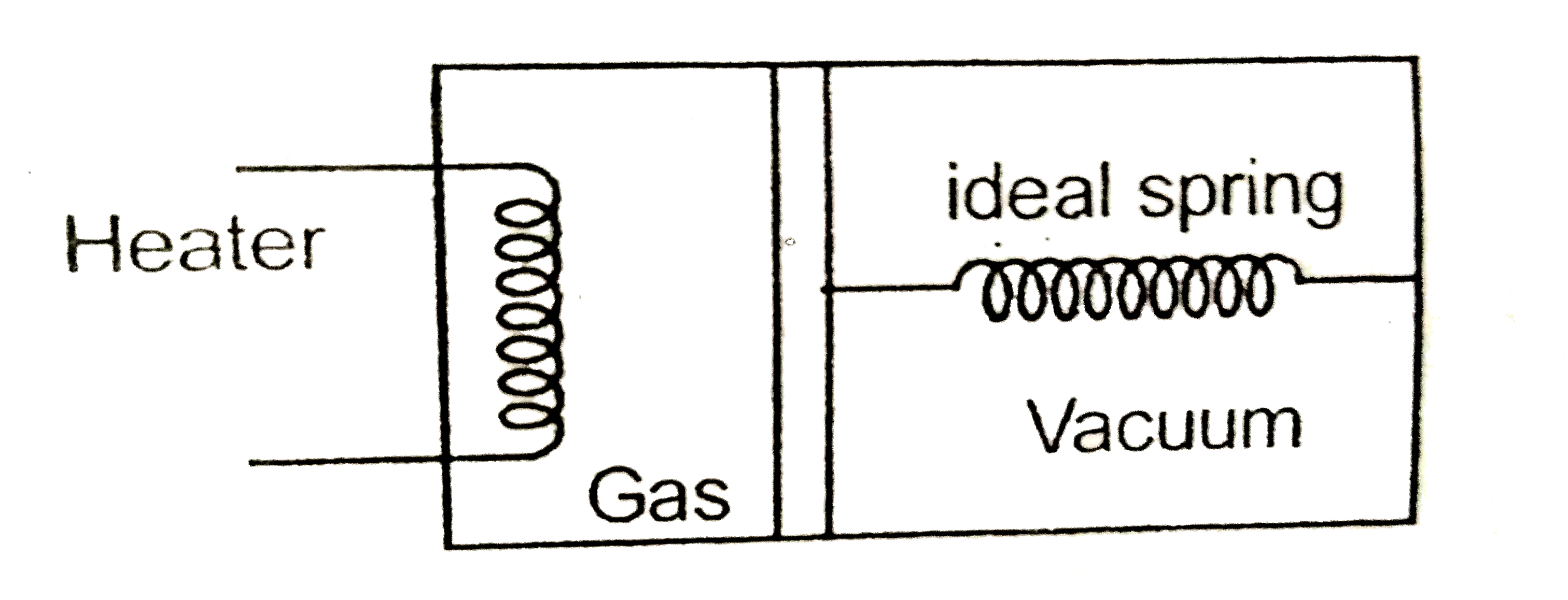
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise TRY YOURSELF|12 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|20 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-I) Subjective Type|5 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-Assignment (Section-J) Aakash Challengers Questions
- The given figrue shows the variation of force exerted on the piston by...
Text Solution
|
- Consider a gas filled in a part of thermally insulated vessel with fri...
Text Solution
|
- A cylinderical vessel made of thermally isulating material is divided ...
Text Solution
|
- A polyatomic ideal gas with linear structure is being supplied that Q ...
Text Solution
|
- Helium gas is taken along the path AtoBtoCtoD as shown the indicator d...
Text Solution
|
- Let F1 be the magnitude of the gravitational force exerted on the Su...
Text Solution
|