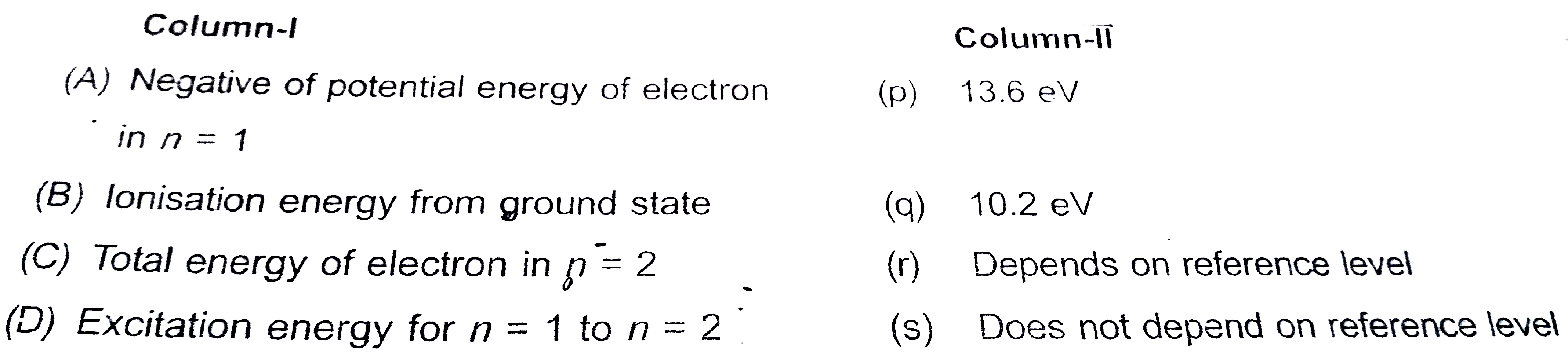
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION G (Integer)|3 VideosATOMS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION H (Multiple True-False)|2 VideosATOMS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION E (Assertion-Reason)|2 VideosALTERNATING CURRENT
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) (Aakash Chailengers Questions)|2 VideosCOMMUNICATION SYSTEMS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION D (Assertion-Reason)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ATOMS-ASSIGNMENT SECTION F (Matrix-Match)
- When we write expression for energy of electron in n^"th" orbit of hyd...
Text Solution
|
- Column-II give some quantities associated with electron of hydrogen li...
Text Solution
|
- In column-I name of the spectral series are given and column-II gives ...
Text Solution
|
- Regarding the spectrum of hydrogen, match the entries in column-I with...
Text Solution
|