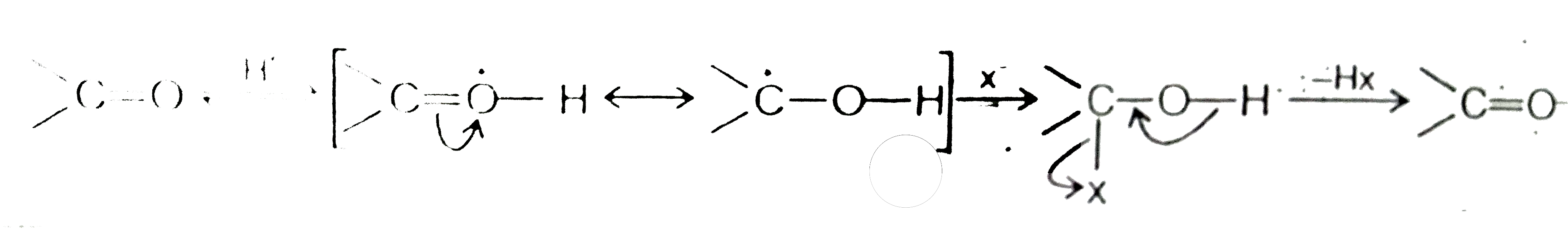
Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Illustrations|3 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section A Competition Level Questions)|60 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -Try Yourself
- Give the reasons for the following: (i) Iodoform is prepared by reac...
Text Solution
|
- The number of molecules in 16g of methane is:
Text Solution
|
- Write the structures of the following compounds (i) 4-chloropentn-2-...
Text Solution
|
- Give chemical equations and name the main product formed when (i) Et...
Text Solution
|
- What are the products formed by reductive ozonolysis of penta-1,3-dien...
Text Solution
|
- Give chemical equations for following conversions: (i) Propanal from...
Text Solution
|
- Identify unknown compounds A to E in the following series of chemical ...
Text Solution
|
- Complete the reaction
Text Solution
|
- Complete the following reactions and identify A, B and C, (i) A+H(2)...
Text Solution
|
- An organic compound [A] with molecular formula C(5)H(8)O(2) is reduced...
Text Solution
|
- Write reactions and conditions to bring about the following conversion...
Text Solution
|
- How can we distinguish chemically the following pairs of compounds? ...
Text Solution
|
- How can you perform the following conversions? Cyclohexanol to Cycl...
Text Solution
|
- Give IUPAC names of the following compounds. (i) HOOC-underset(OH)un...
Text Solution
|
- At STP 5.6 "litre" of a gas weigh 8 g. The vapour density of gas is:
Text Solution
|
- A compound [A] of molecular formula C(4)H(9)Br yields a compound [B] o...
Text Solution
|
- Calculate the frequency of a photon, having energy 41.25 eV. (h=6.6xx1...
Text Solution
|
- How will you convert benzene into
Text Solution
|
- Arrange the following in decreasing order of nucleophilic addition C...
Text Solution
|
- The activation energies of the forward and backward reactions in the c...
Text Solution
|
- Ethyl alcohol is heated with conc. H(2)SO(4) at 170^(@)C. The product ...
Text Solution
|