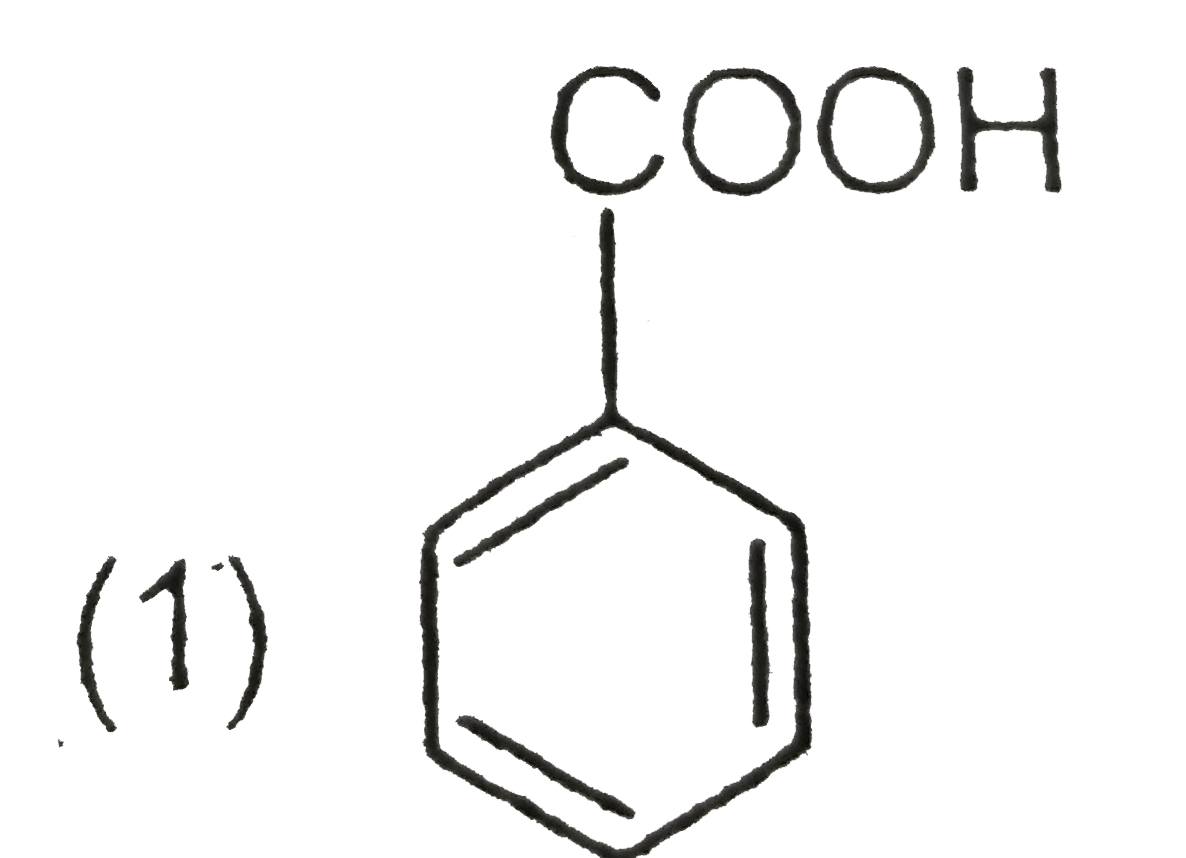
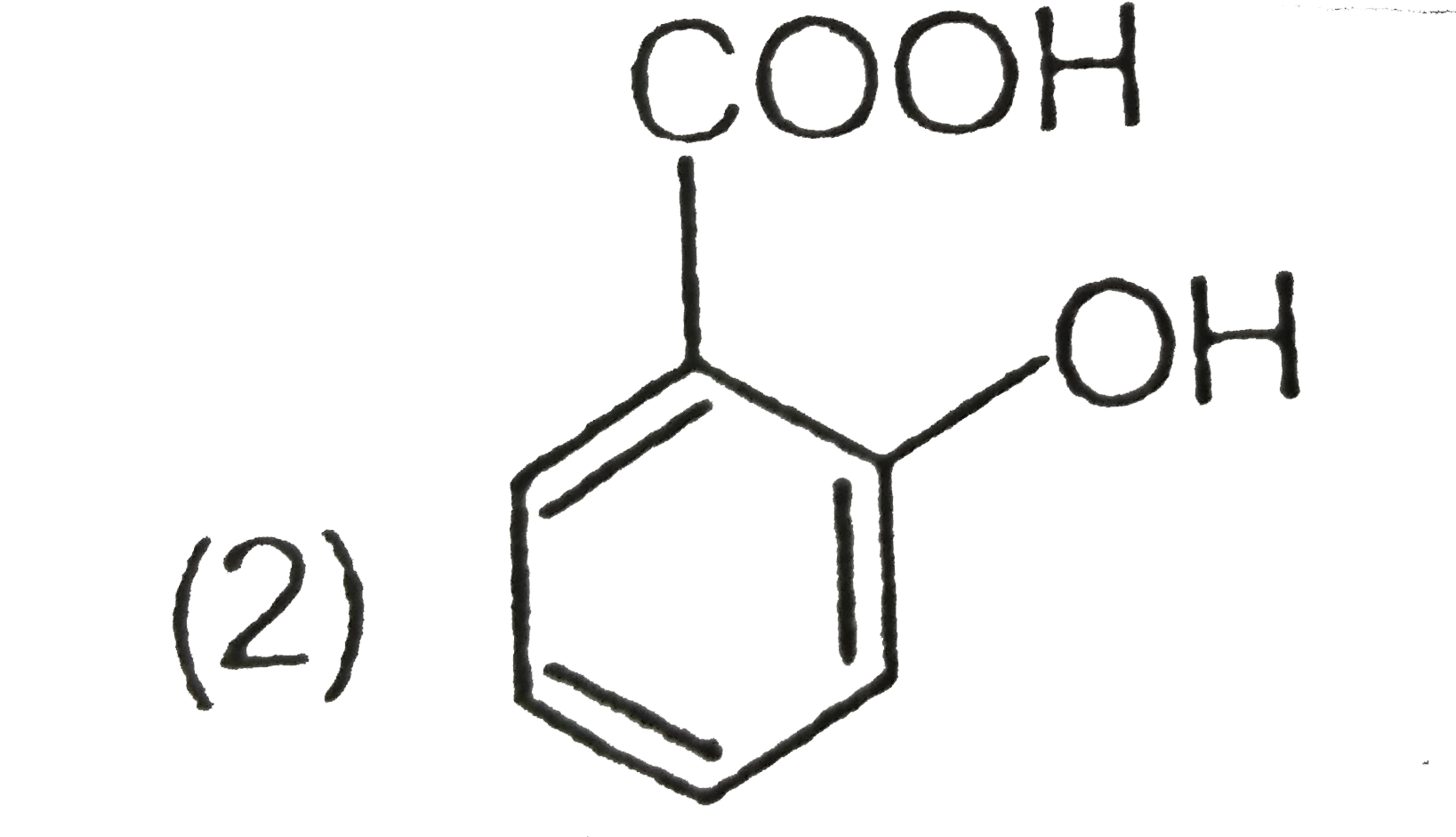
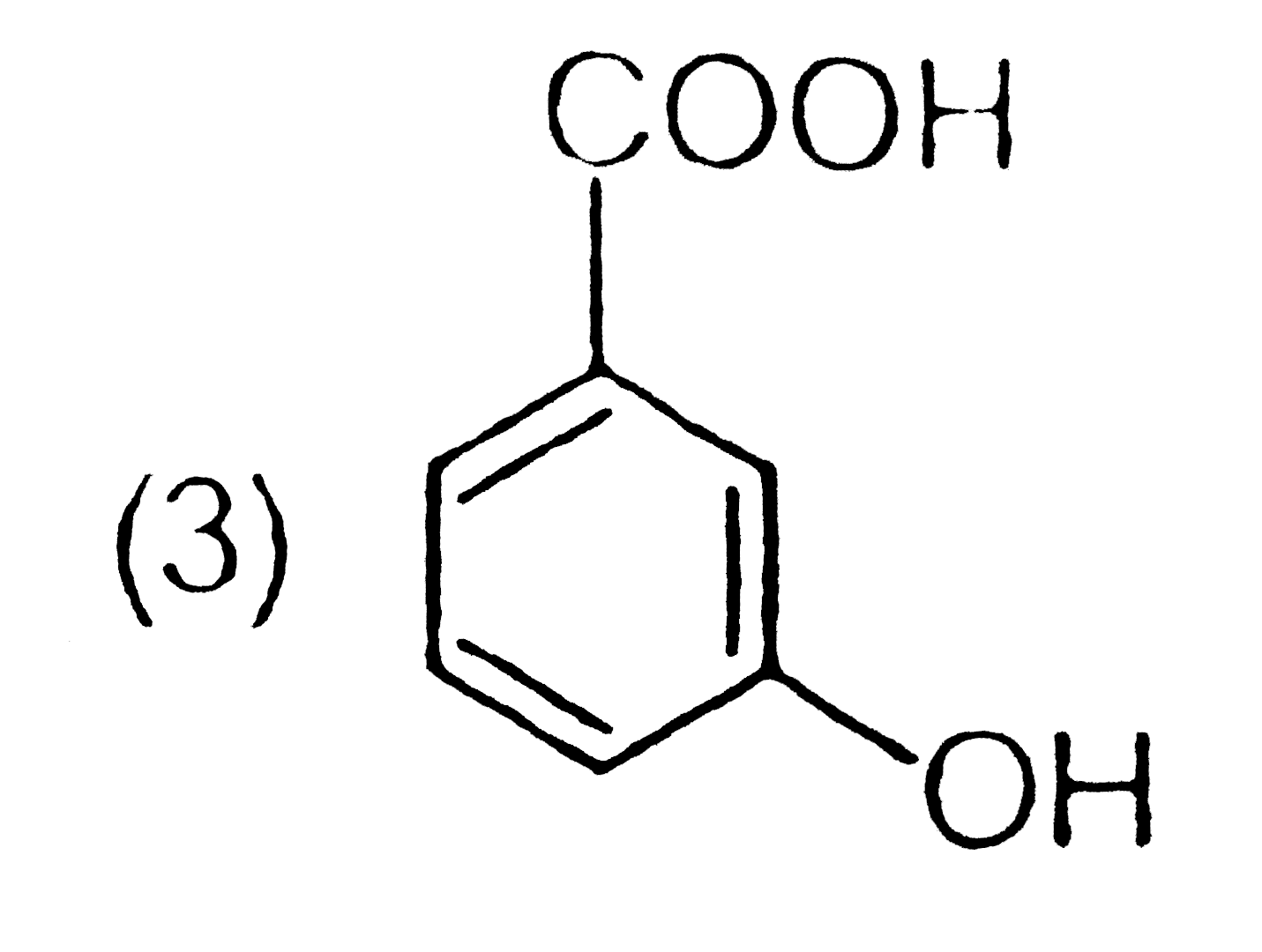
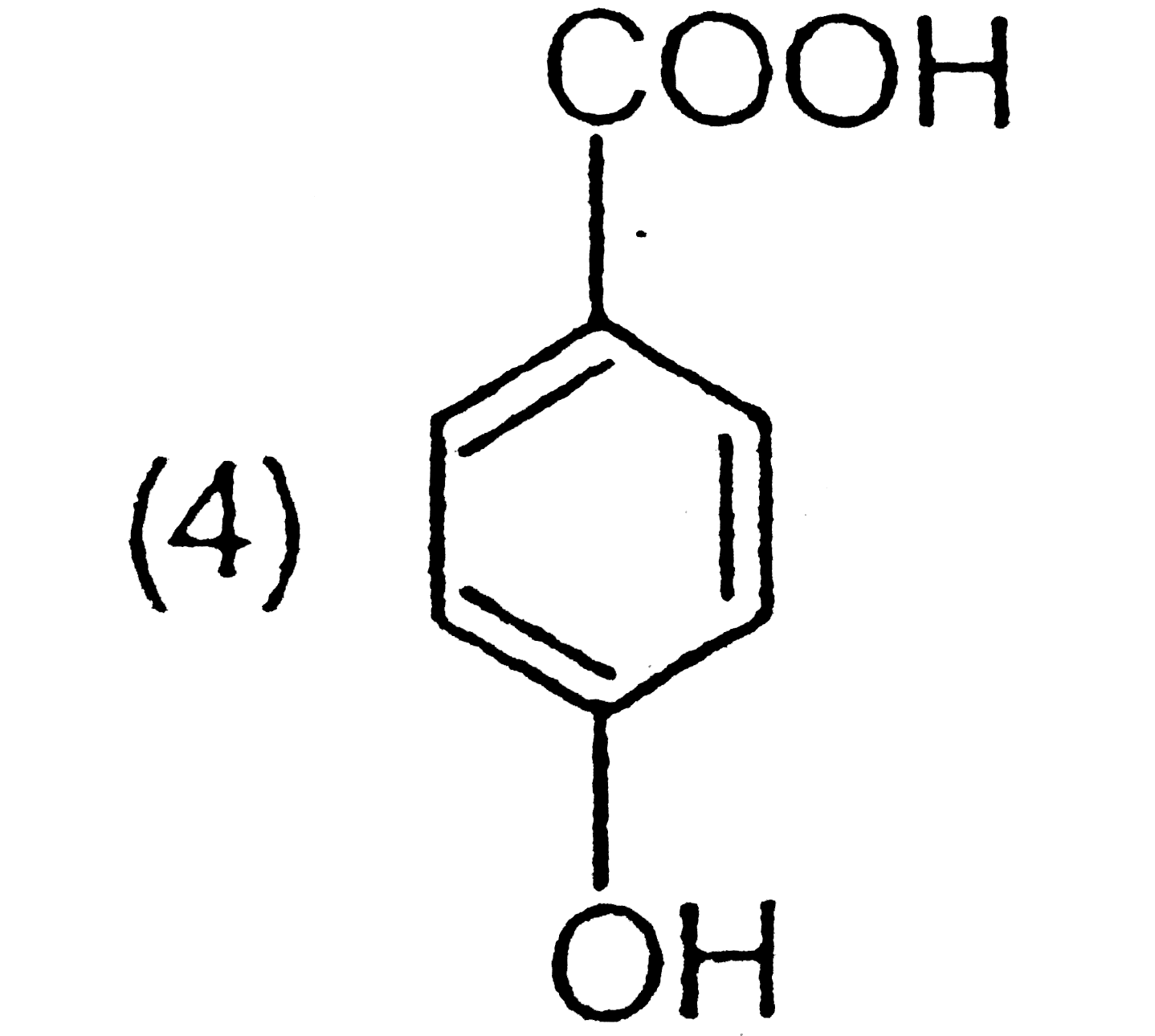
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -B OBJECTIVE TYPES QUESTIONS (ONE OPTION IS CORRECT)|24 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -C Objective type questions more than one options are correct)|11 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Illustrations|3 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -Assignment (Section A Competition Level Questions)
- The diketone CH(3)-overset(overset(O)(||))(C)-(CH(2))(2)-overset(over...
Text Solution
|
- What will be the product , when carboxy phenol, obtained by Reimer Tie...
Text Solution
|
- Which of the following aromatic acids is most acidic?
Text Solution
|
- CH(3)COOH overset (Delta) underset (P(2)O(5)) to X. Identify X
Text Solution
|
- Which one of the following orders of acidic strength is correct?
Text Solution
|
- Which of the following represents the correct order of acidity in the ...
Text Solution
|
- The correct order of acidic strength is
Text Solution
|
- Treatment of benzoic acid with Cl(2)//FeCl(3) will give
Text Solution
|
- In esterfication
Text Solution
|
- Acetyl chloride is reduced with LiAlH(4) , the product formed is
Text Solution
|
- The reaction CH(3) COOH + Cl(2) overset (red P) to CICH(2) COOH + HC...
Text Solution
|
- Saponification of ethyl benzoate with caustic soda as alkali gives
Text Solution
|
- Which of the following acids has the smallest dissociation constant?
Text Solution
|
- Which of the following compounds will show the maximum ‘enol’ content?
Text Solution
|
- Aliphatic aldehyde can be oxidised by
Text Solution
|
- Formaldehyde when treated with KOH (caustic potash) gives methanol and...
Text Solution
|
- Reactions
Text Solution
|
- Bakelite is prepared by the reaction between
Text Solution
|
- Fehling test is positive for
Text Solution
|
- 2C(6)H(5)CHO overset(NaOH) to C(6)H(5)CH(2)OH + C(6)H(5)COONa The si...
Text Solution
|