Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-H) (Multiple True-false Type questions)|3 VideosKINETIC THEORY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-I) (Subjective Type Questions)|5 VideosKINETIC THEORY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-F) (Matrix-match type questions)|3 VideosGRAVITATION
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION - D (ASSERTION-REASON TYPE QUESTIONS)|15 VideosLAWS OF MOTION
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION-D) (Assertion-Reason Type Questions)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-KINETIC THEORY-Assignment (Section-G) (Integer Answer Type Question)
- A gaseous mixture enclosed in a vessel consists of 1 g mole of a gas A...
Text Solution
|
- Find the rotational kinetic energy of 2 kg of oxygen gas molecules (in...
Text Solution
|
- A uniform tube closed at one end, contains a pallet of mercury 4 cm lo...
Text Solution
|
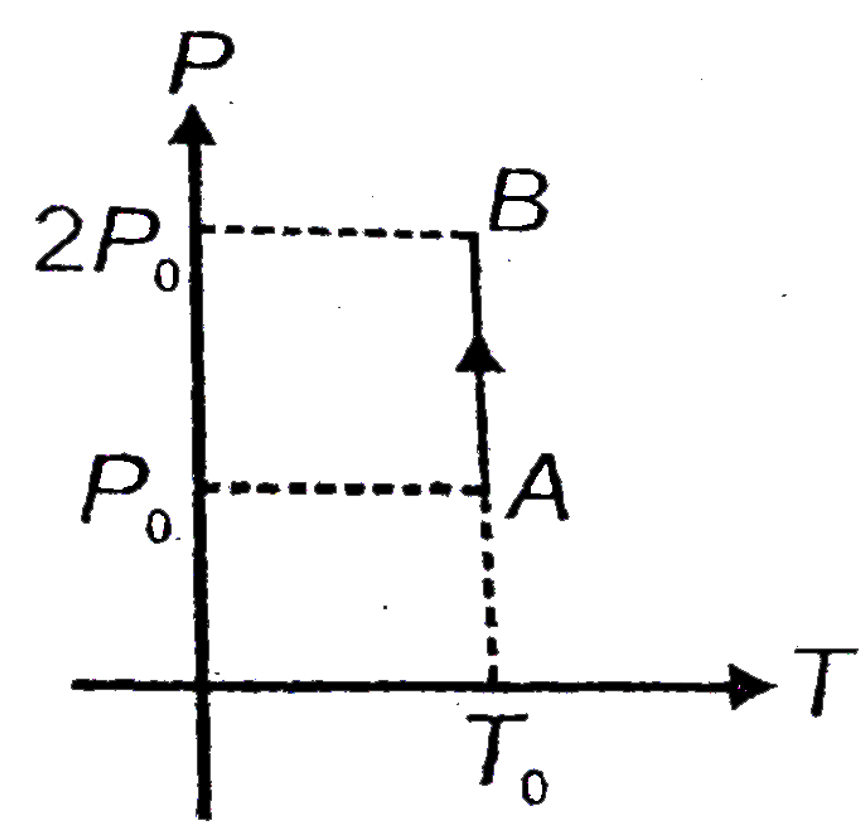
- For a given mass of gas the variation of pressure versus temperature i...
Text Solution
|
 .
.