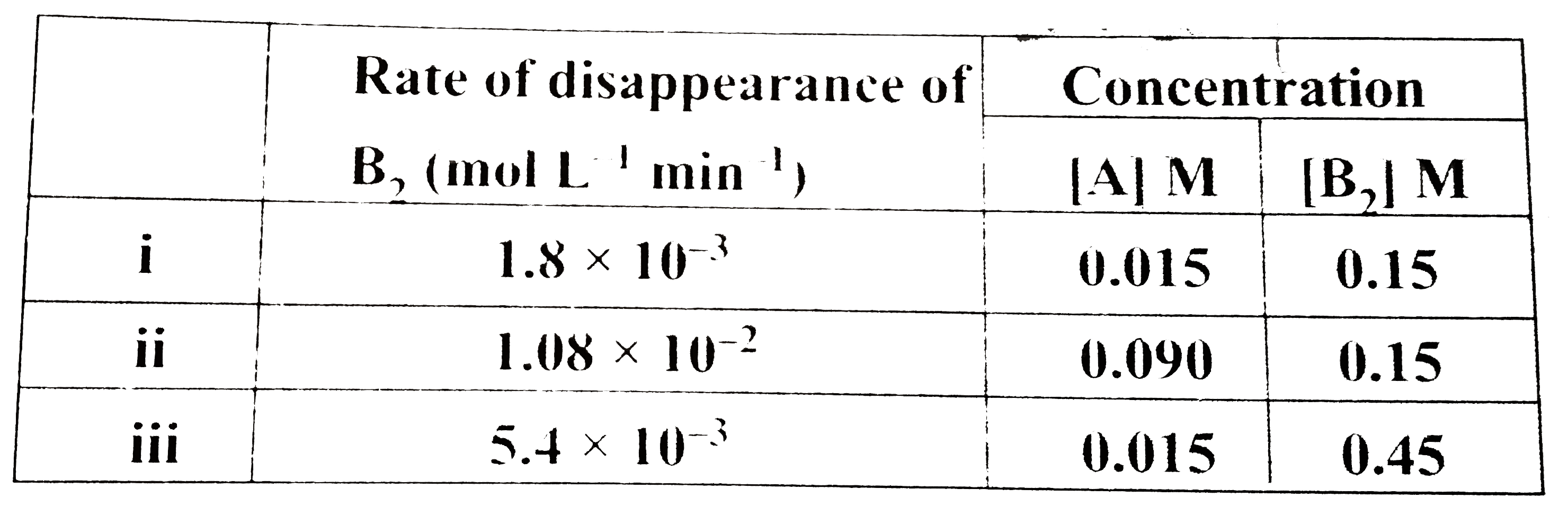A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION C : Previous Year Questions)|62 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION A : Objective Type Questions)|50 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH INSTITUTE ENGLISH|Exercise Assignment ( SECTION - A)|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-CHEMICAL KINETICS-ASSIGNMENT (SECTION B : Objective Type Questions)
- Consider the data given below for hypothetical reaction A rarr X {:...
Text Solution
|
- The graph between log k versus 1//T is a straight line.
Text Solution
|
- The temperature coefficient of most of the reactions lies between
Text Solution
|
- If the volume of the vessel in which the reaction 2NO+O2 rarr2NO2 is...
Text Solution
|
- For the raction A+Brarr products, it is found that order of A is 2 and...
Text Solution
|
- Nitric oxide (NO) reacts with oxygen to produce nitrogen dioxide 2NO...
Text Solution
|
- For the first order reaction, the taken to reduce the initial concentr...
Text Solution
|
- The rate of the reaction 2N(2)O(5) to 4NO(2) + O(2) can be written...
Text Solution
|
- For the reaction , N2O4(g)underset(K2)overset(K1)hArr2NO2(g), the rat...
Text Solution
|
- For a homogeneous gaseous reaction A rarr B + C + D , the initial pres...
Text Solution
|
- form the gaseous reaction 2A + B(2) rarr 2AB, the following rate dat...
Text Solution
|
- The inversion of a sugar follows first order rate equation which can b...
Text Solution
|
- Which of the following is correct
Text Solution
|
- The rate constant for a reaction is 1.5 xx 10^(-7) at 50^(@)C and 4.5...
Text Solution
|
- In Arrhenius equation , k=Ae^(Ea/(RT)) , A may be termed as rate const...
Text Solution
|
- The rate constant of the production of 2B (g) by the reaction , A(g)ov...
Text Solution
|
- Two substances A and B are present such that [A(0)]=4[B(0] and half-l...
Text Solution
|
- If the rate of reaction increases by 27 times , when temperature is in...
Text Solution
|
- the reaction A to B follows first order Kinetics the time taken...
Text Solution
|
- If find B%
Text Solution
|