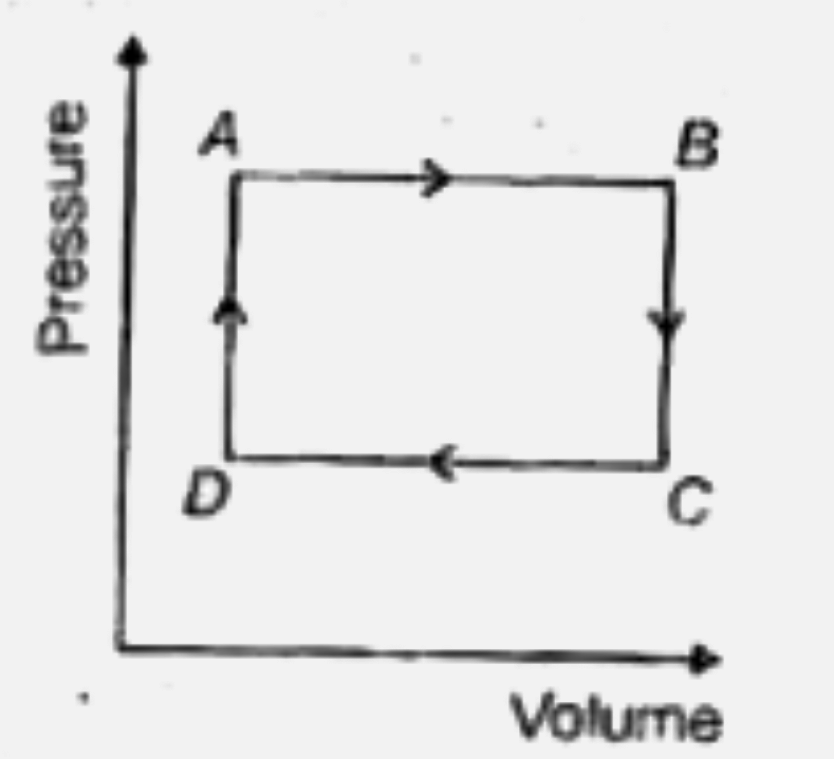
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The pressure and volume of a gas are changed as shown in the p-V diagr...
Text Solution
|
- A P-V diagram is obtained by changing the temeprature of the gas as sh...
Text Solution
|
- In the diagrams (i) to (iv) of variation of volume with changing press...
Text Solution
|
- The pressure and volume of gas are changed as shown in the P-V diagram...
Text Solution
|
- The Volume of one mole of the gas is changed from V to 2V at constant ...
Text Solution
|
- The pressure and volume of a gas changed as shown in the P-V diagram i...
Text Solution
|
- The variation of pressure P with volume V for an ideal monatomic gas d...
Text Solution
|
- An ideal gas of volume V and pressure P expands isothermally to volume...
Text Solution
|
- p and V are pressure and volume respectively of a gas of a particular ...
Text Solution
|