Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
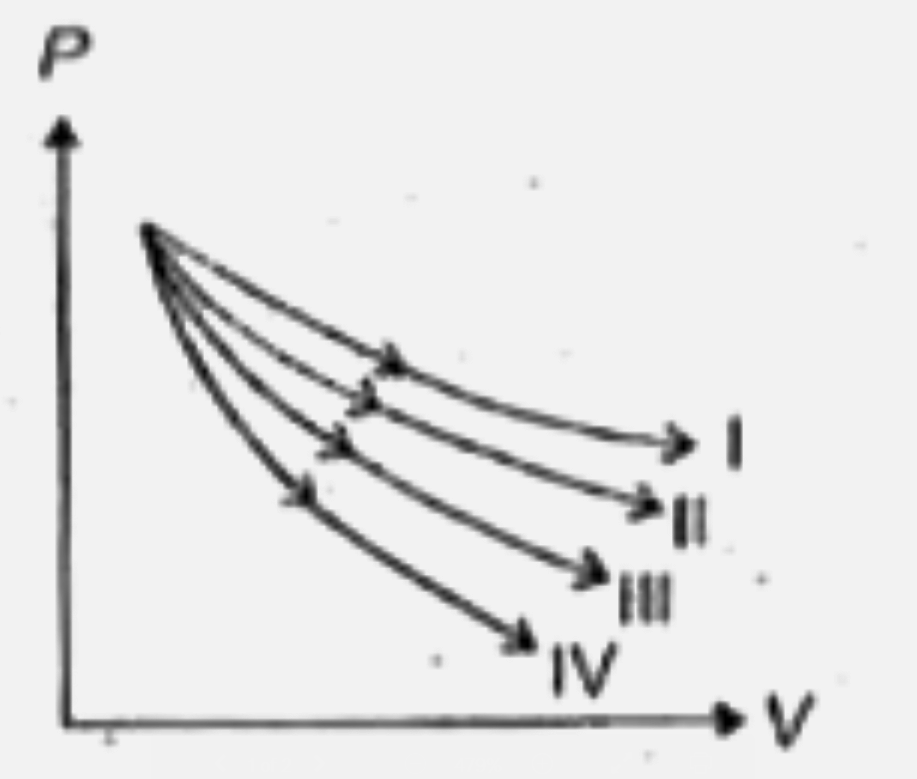
- In the following plots match I,II, III,Iv with (a) isothermal process ...
Text Solution
|
- PV = n RT holds good for A) Isobaric process B) Isochoric process C) I...
Text Solution
|
- Thermodynamic processes are indicated in the following diagrams Match ...
Text Solution
|
- Consider the following cyclic process. I. Isothermal , II. Adiabatic ,...
Text Solution
|
- Thermodynamic processes are indicated in the following diagram. M...
Text Solution
|
- {:((1),"Mono atomic molecule",(a),1.33),((2),"Diatomic molecule (Norma...
Text Solution
|
- Define the following terms: (a) Isothermal process (b) adiabatic proce...
Text Solution
|
- Define the following terms: (a) Isothermal process (b) adiabatic proce...
Text Solution
|
- Explain (i) Reversible and irreversible process (ii) Isothermal an...
Text Solution
|