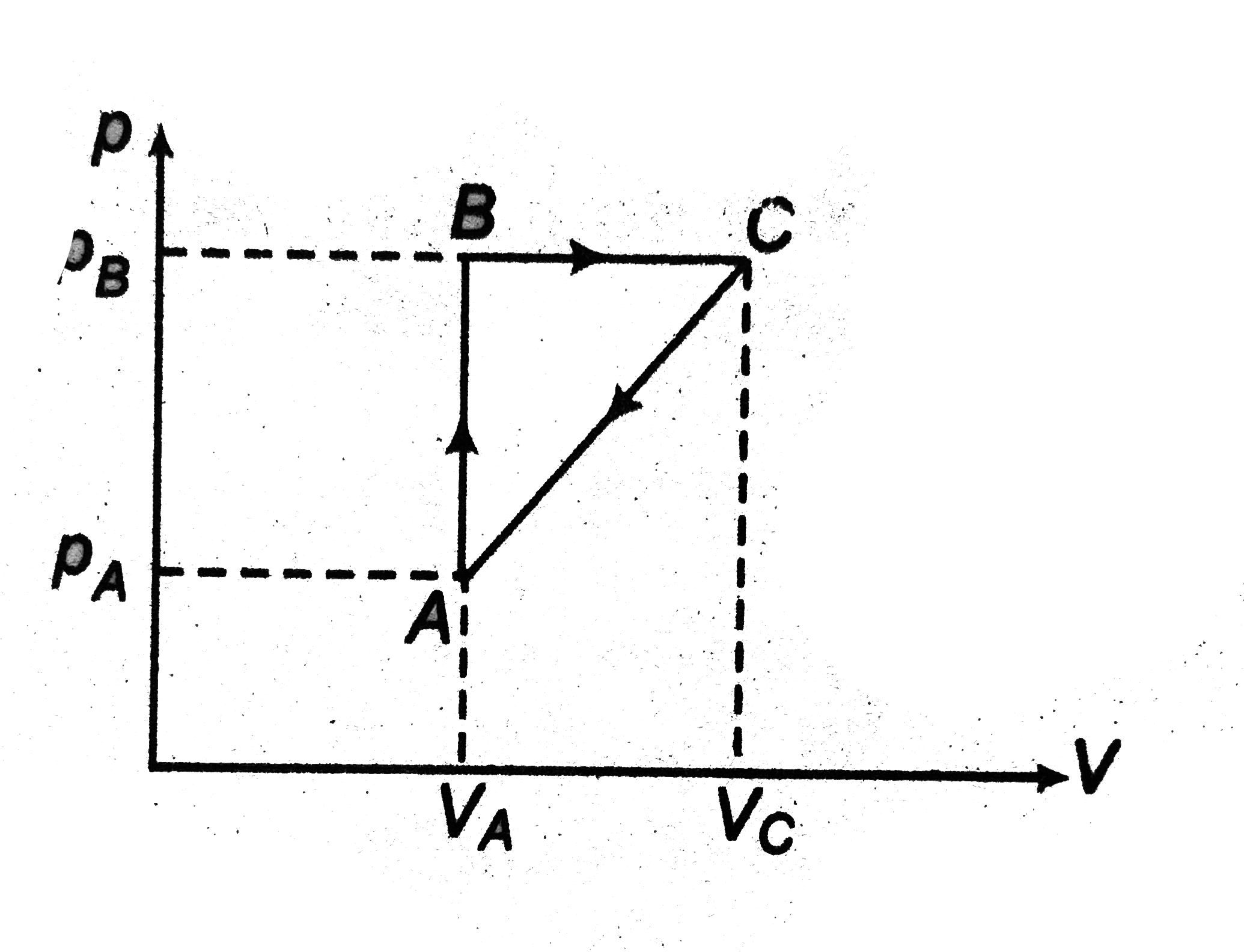A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A thermodynamical process is shown in the figure with pA=3xxp(atm), VA...
Text Solution
|
- A thermodynamical process is shown in the figure with pA=3xxp(atm) , V...
Text Solution
|
- 1.0 k-mol of a sample of helium gas is put through the cycle of operat...
Text Solution
|
- A thermodynamic process is shown in the following figure. The process ...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- A thermodynamic process is shown in the following figure. In the proce...
Text Solution
|
- A cyclic process contains four steps AB,BC,CD and DA. Heat involved in...
Text Solution
|
- चित्र में प्रदर्शित ऊष्मीय प्रक्रम में, PA=3xx10^4 "न्यूटन//मीटर"^2 ...
Text Solution
|