A
B
C
D
Text Solution
Verified by Experts
Recommended Questions
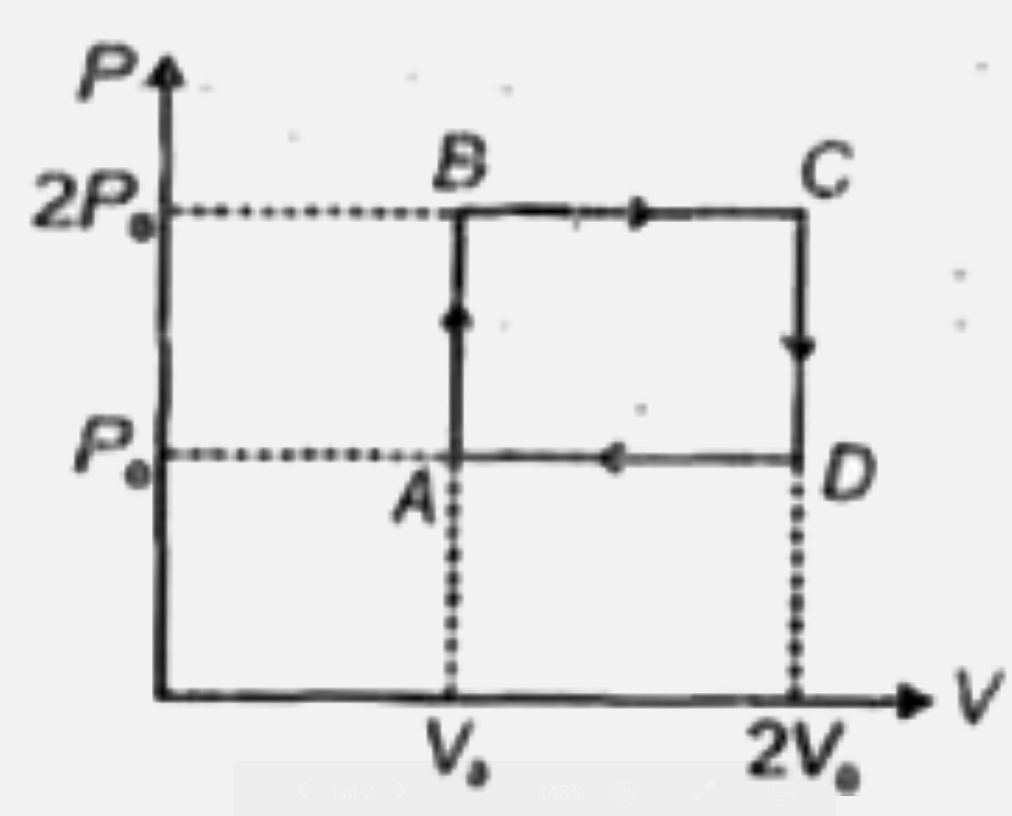
- N moles of a monotomic gas is carried round the reversible rectangular...
Text Solution
|
- An ideal monatomic gas is taken round the cycle ABCDA as shown in the ...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- An ideal monoatomic gas is carried around the cycle ABCDA as shown in ...
Text Solution
|
- N moles of a monoatomic gas is carried round the reversible rectangula...
Text Solution
|
- An ideal monoatomic gas is carried around the cycle ABCDA as shown in ...
Text Solution
|
- एक आदर्श एकपरमाणुक गैस का चक्र ABCDA के चारों ओर ले जाया जाता है जैसा ...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- दो मोल हीलियम गैस चक्र ABCDA के लिये ली गई है जैसा P-T ग्राफ में प्रदर...
Text Solution
|