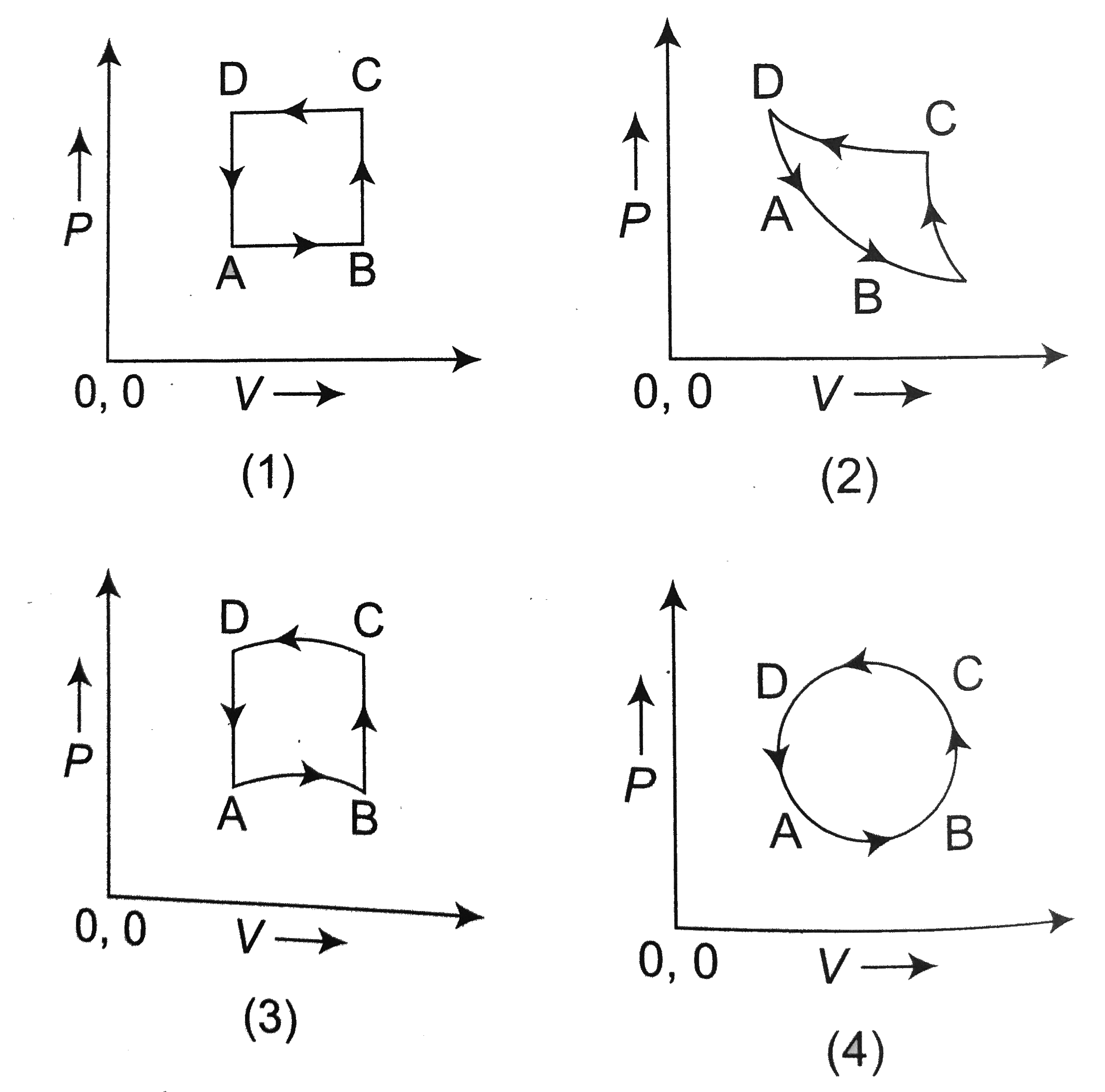
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -B) (Objective Type Questions)|30 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -C) (Previous Year Questions)|63 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|20 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-ASSIGNMENT (SECTION -A) (Objective Type Questions)
- Select the incorrect statement about the specific heat of a gaseous sy...
Text Solution
|
- Work done in the cyclic process shown in figure is
Text Solution
|
- In following figs. Variation of volume by change of pressure is shown ...
Text Solution
|
- In a thermodynamic process, pressure of a fixed mass of a gas is chang...
Text Solution
|
- Figure shows two processes a and b for a given sample of gas. If Delta...
Text Solution
|
- A gas undergoes a change at constant temperature. Which of the followi...
Text Solution
|
- Following figure shows P-T graph for four processes A, B, C and D.Sele...
Text Solution
|
- An ideal gas with adiabatic exponent gamma is heated at constant press...
Text Solution
|
- If vec a and vec b are two unit vectors and theta is the angle between...
Text Solution
|
- In the diagram shown Q(iaf)= 80 cal and W(iaf)= 50 cal. If W = - 30 ca...
Text Solution
|
- A mass of dry air at N.T.P. is compressed to (1)/(32) th of its origin...
Text Solution
|
- Two sample A and B of a gas initially at the same pressure and tempera...
Text Solution
|
- The adiabatic elasticity of hydrogen gas (gamma=1.4) at NTP
Text Solution
|
- For an isomertric process
Text Solution
|
- A mixture of gases at NTP for which gamma = 1.5 si suddenly compressed...
Text Solution
|
- In which process P-V diagramis a stright line parallel to the volume a...
Text Solution
|
- p-V plots for two gases during adiabatic process as shown in figure pl...
Text Solution
|
- The pressure and volume of a gas are changed as shown in the p-V diagr...
Text Solution
|
- The figure shows P-V diagram of a thermodynamic cycle. Which correspon...
Text Solution
|
- During the thermodynamic process shown in figure for an ideal gas
Text Solution
|