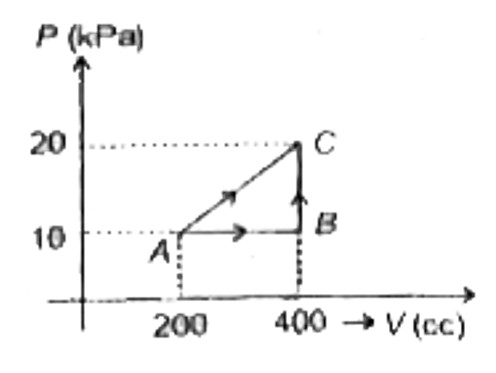
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -C) (Previous Year Questions)|63 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -D) (Assertion - Reason Type Questions)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -A) (Objective Type Questions)|47 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-ASSIGNMENT (SECTION -B) (Objective Type Questions)
- An ideal monatomic gas at 300 K expands adiabatically to 8 times its v...
Text Solution
|
- Slope of isotherm for a gas (having gamma = (5)/(3)) is 3 xx 10^(5) N/...
Text Solution
|
- A gas may expand either abiabatically or isothermally .A number of P...
Text Solution
|
- The variation of pressure P with volume V for an ideal monatomic gas d...
Text Solution
|
- Figure shows , the adiabatic curve on log T and log V scale performed ...
Text Solution
|
- A cyclic process on an ideal monatomic gas is shown in figure . The co...
Text Solution
|
- A diatomic gas undergoes a process represented by PV^(1.3)= constant ....
Text Solution
|
- If a gas is taken from A to C through B then heat absorbed by the gas ...
Text Solution
|
- The process CD is shown in the diagram . As system is taken from C to ...
Text Solution
|
- A. P . T graph is shown for a cyclic process . Select correct statemen...
Text Solution
|
- A hydrogen cylinder is designed to withstand an internal pressure of 1...
Text Solution
|
- An ideal gas of volume V and pressure P expands isothermally to volume...
Text Solution
|
- The pressure P of an ideal diatomic gas varies with its absolute tempe...
Text Solution
|
- An ideal gas expands according to the law P^(2) V = constant . The in...
Text Solution
|
- The variation of pressure P with volume V for an ideal diatomic gas is...
Text Solution
|
- Neon gas of a given mass expands isothermally to double volume . What ...
Text Solution
|
- When 1 kg of ice at 0^(@)C melts to water at 0^(@)C, the resulting cha...
Text Solution
|
- Carrot cycle is plotted in P-V graph . Which portion represents an iso...
Text Solution
|
- Efficiency of a heat engine working between a given source and sink is...
Text Solution
|
- A heat engine rejects 600 cal to the sink at 27^(@)C . Amount of work ...
Text Solution
|