A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -D) (Assertion - Reason Type Questions)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -B) (Objective Type Questions)|30 VideosTHERMAL PROPERTIES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J) Akash Challengers Questions|7 VideosUNITS AND MEASUREMENTS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-ASSIGNMENT (SECTION -C) (Previous Year Questions)
- One mole of an ideal gas goes form an initial stateA to final state B...
Text Solution
|
- A thermodynamics system is taken trough the cycle ABCD as shown in fig...
Text Solution
|
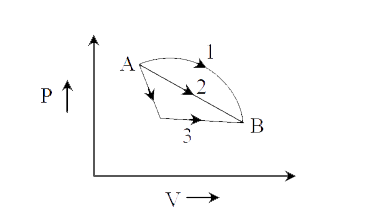
- An ideal gas goes from state A to state B via three different processe...
Text Solution
|
- During an isothermal expansion , a confined ideal gas does -150J of wo...
Text Solution
|
- A mass of diatomic gas (gamma = 1.4) at a pressure of 2 atmosphere is ...
Text Solution
|
- If Delta U and Delta W represent the increase in internal energy and w...
Text Solution
|
- If C(p) and C(v) denote the specific heats (per unit mass) of an ideal...
Text Solution
|
- A monoatomic gas at pressure P(1) and volume V(1) is compressed adiaba...
Text Solution
|
- In thermodynamic processes which of the following statements is not tr...
Text Solution
|
- The internal energy change in a system that has absorbed 2 kcal of hea...
Text Solution
|
- If Q, E and W denote respectively the heat added, change in internal e...
Text Solution
|
- At 10^(@)C the value of the density of a fixed mass of an ideal gas di...
Text Solution
|
- The efficiency of a heat engine is 1//6Its efficiency double when the ...
Text Solution
|
- A Carnot engine whose sink is at 300K has an efficiency of 40%. By how...
Text Solution
|
- The molar specific heat at constant pressure of an ideal gas is (7/2)R...
Text Solution
|
- Which of the following processes is reversible
Text Solution
|
- An ideal gas heat engine operates in Carnot cycle between 227^(@)C and...
Text Solution
|
- A system is taken from state a to state c by two paths abc and abc as ...
Text Solution
|
- Consider two insulated chambers (A,B) of same volume connected by a cl...
Text Solution
|
- A Carnot engine has efficiency 25%. It operates between reservoirs of ...
Text Solution
|