Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
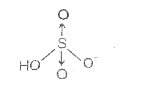
- Calculate the formal charge on S in following structure :
Text Solution
|
- Application Of Formal Charge To Resonance Structure
Text Solution
|
- In the following electron dot structure, calculate the formal charge f...
Text Solution
|
- Calculate the formal charge on S in HSO(4)^(-) ion
Text Solution
|
- What is the formal charge on carbon atom in the following two structur...
Text Solution
|
- Calculate formal charge (F) on nitrogen and Oxygen atoms marked 'a' an...
Text Solution
|
- Calculate the formal charge on each in the following electron-dot stru...
Text Solution
|
- Formal charge,Resonance and Resonating structures
Text Solution
|
- Calculate the formal charge on S in following structure :
Text Solution
|