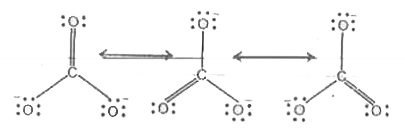
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the resonance structures of CO(3)^(2-)" and "HCO(3)^(-).
Text Solution
|
- Explain the structure of CO(3)^(2-) ion in terms of resonance (b) Ex...
Text Solution
|
- Write the resonance structure of CO(3)^(2-) and HCO(3)^(ө).
Text Solution
|
- CO(3)^(2-) and HCO(3)^(-) can be distingusihed by:
Text Solution
|
- CO(3)^(2-) तथा HCO(3)^(-) की अनुनादी संरचनाए लीखिए ।
Text Solution
|
- Write the reasonance structure of CO(3)^(2-) and HCO(3)^(-) .
Text Solution
|
- O(3) तथा CO(2) की अनुनादी संरचनाएँ लिखिए ।
Text Solution
|
- CO(3)^(2-) तथा "HCO"(3)^(-) की अनुनादी संरचनाए लिखिए |
Text Solution
|
- Write the resonating structures for the following molecules. CO(3)^(...
Text Solution
|