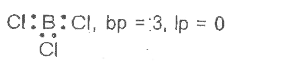Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
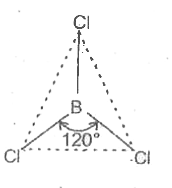
- Discuss the shape of the B Cl(3) molecules using VSEPR model .
Text Solution
|
- Discuss the shape of the BeF(2) molecules using VSEPR model
Text Solution
|
- Discuss the shape of the IF(5) using VSEPR model .
Text Solution
|
- Discuss the shape of the B Cl(3) molecules using VSEPR model .
Text Solution
|
- Discuss the shape of the SiF(4) molecule using VSEPR model .
Text Solution
|
- Discuss the shape of H(3)O^(+) using VSEPR model .
Text Solution
|
- Discuss the shape of RF(4)^(-) using VSEPR model
Text Solution
|
- Discuss the shape of the BeF(2) molecules using VSEPR model .
Text Solution
|
- Discuss the shapes of following molecules using VSEPR model : (i) SiCl...
Text Solution
|