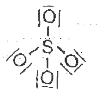
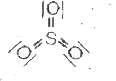
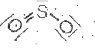A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- In the presence of a catalyst, the activation energy is lowered by 3 K...
Text Solution
|
- Which of the following structures is the most perferred and hence of l...
Text Solution
|
- Which of the following oxide is the most acidic in nature? Al(2)O(3),S...
Text Solution
|
- Which of the following structures is the most preferred and hence of l...
Text Solution
|
- Which of the following is the most preferred conformation of ethane mo...
Text Solution
|
- SO(3) के अधिशोषण के लिए सर्वाधिक उपयुक्त है
Text Solution
|
- SO(3) के लिए निम्न में से कौन सबसे अधिक स्वीकार्य और इसलिए निम्नतम् ऊर...
Text Solution
|