A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section - C) Previous Years Questions|60 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section - A) Objective Type Questions|55 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-ASSIGNMENT (Section - B) Objective Type Questions
- The standard heat of formation of NO(2)(g) and N(2)O(4)(g) are 8.0 an...
Text Solution
|
- If (1)/(2)X(2)O((s)) rarr X((s))+(1)/(4)O(2(g)),DeltaH=90kJ then heat ...
Text Solution
|
- For a gaseous reaction A(g) + 3 B(g) rightarrow 3C(g) + 3D(g) Delt...
Text Solution
|
- A mixture of 2 moles of CO and 1 mole of O(2), in a closed vessele is ...
Text Solution
|
- The bond dissociation energies of X2, Y2 and XY are in the ratio of 1 ...
Text Solution
|
- Vapour density of a gas is 8. Its molecular mass will be
Text Solution
|
- If x mole of ideal gas at 27^(@)C expands isothermally and reversibly ...
Text Solution
|
- Enthalpy of formation of NH(3) is -X kJ and Delta H(H-H), Delta H(N-H)...
Text Solution
|
- A system X undergoes following changes underset(P(1)V(1)T(1))(X)to u...
Text Solution
|
- The heat of neutralisation for strong acid and strong base forming 2 m...
Text Solution
|
- The value of Delta H^(@) in kJ for the reaction will be CS(2)(l) + 4NO...
Text Solution
|
- The heat librerated on complete combustion of 1 mole of CH(4) gas to C...
Text Solution
|
- The work done in an open vessel at 300K, when 112g iron reacts with di...
Text Solution
|
- Which statement is correct?
Text Solution
|
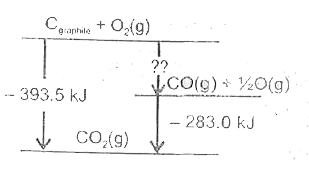
- A schematic representation of enthalpy changes for the reaction, C("gr...
Text Solution
|
- Which of the following equations respresents standard heat of formatio...
Text Solution
|
- Different types of systems are given below The A and B systems re...
Text Solution
|
- Set of intensive properties is shown by
Text Solution
|
- For the expansion occuring from initial to final stage in finite time,...
Text Solution
|
- Calorific value of ethane, in k J/g if for the reaction 2C(2)H(6) + 7O...
Text Solution
|