Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
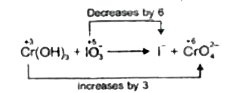
- Balance the ionic equation in alkaline medium Cr(OH)(3) + IO(3)^(-)...
Text Solution
|
- Balance the following reaction by oxidation number method: CrO(4)^(2...
Text Solution
|
- Balance the following by ion electron method (basic medium): Cr(OH)(...
Text Solution
|
- In the following reaction: Cr(OH)(3)+OH^(-)+IO(3)^(-) toCrO(4)^(2-)+...
Text Solution
|
- Balance the following equation by oxidation number method. Cl(2)+IO(...
Text Solution
|
- निम्नलिखित समीकरण को ऑक्सीकरण संख्या विधि के द्वारा सन्तुलित करो- I(...
Text Solution
|
- निम्नलिखित समीकरण को क्षारीय माध्यम में संतुलित कीजिए - Cr(OH)(3)+I...
Text Solution
|
- निनलिखित समीकरण को ऑक्सीकरण संख्या विधि से संतुलित कीजिए - CrO(4)^(2...
Text Solution
|
- Write the ionic equation of the disproportionation reaction of CrO(4)^...
Text Solution
|