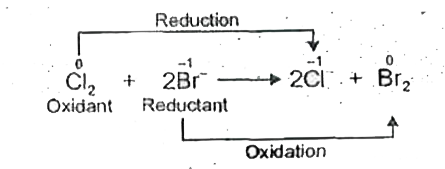
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Identify the oxidant and the reductant respectively in the following r...
Text Solution
|
- For the reaction : Cl(2)(aq) +2Br^(-) (aq) to Br(2) (aq) +2Cl^(-)(aq) ...
Text Solution
|
- The standard potential for the reaction, Cl(2)(g) + 2Br^(-)(aq) righ...
Text Solution
|
- Identify the oxidant the reductant in the following reaction. Cl(2)(g)...
Text Solution
|
- निम्नलिखित अभिक्रयाओं में ऑक्सीकरण तथा अपचायक को पहचानिए - (i) Cl(2)...
Text Solution
|
- Bromine is prepared commercially by the reaction : 2Br^(-)(aq)+Cl(2)...
Text Solution
|
- Write the expression of K(c) for the reactions: Cl(2)(g)+2Br^(-)(aq)hA...
Text Solution
|
- The reaction Cu^(2+)(aq)+2Cl^(c-)(aq) rarr Cu(s)+Cl(2)(g) has E^(c-).(...
Text Solution
|
- In the following redox reactions, identify the oxidation half -reactio...
Text Solution
|