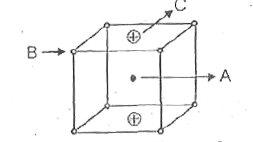
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - B) (OBJECTIVE TYPE QUESTION)|25 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - C) (PREVIOUS YEARS QUESTION)|41 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|30 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THE SOLID STATE -Assignment (SECTION - A) (OBJECTIVE TYPE QUESTION)
- A compound formed by element A and B crystallize in the cubic structur...
Text Solution
|
- A solid with formula ABC(3) would probably have
Text Solution
|
- A solid ABC has A, B and C arranged as below. The formula of solid is
Text Solution
|
- An alloy of copper silver and gold is found it have copper constitutin...
Text Solution
|
- In a face centred cubic arrangement of A and B atoms whose A atoms are...
Text Solution
|
- In any ionic crystal A has formed cubical close packing and B atoms ar...
Text Solution
|
- A binary solid A^(+)B^(-) has a structure with B^(-) ions constituting...
Text Solution
|
- In a crystalline solid, anion B are arranged in cubic close packing an...
Text Solution
|
- In a cubic close packed structure of mixed oxides, the lattice is made...
Text Solution
|
- Titanium crystallizes in a face centred cubic lattice. It reacts with ...
Text Solution
|
- The site labelled as 'X' in fcc arrangement is
Text Solution
|
- A unit cell is obtained by closed packing layers of atoms in ABAB …… p...
Text Solution
|
- In certain solid, the oxide ions are arranged in ccp. Cations A occupy...
Text Solution
|
- A solid has a structure in which A atoms are located at the cube corne...
Text Solution
|
- If a is the length of unit cell, then which one is correct relationshi...
Text Solution
|
- For face centered cubic structure edge length 'a' can be related with ...
Text Solution
|
- A crystalline solid AB adopts sodium chloride type structure with edge...
Text Solution
|
- If radius of an octahedral void is r and atomic radius of atoms assumi...
Text Solution
|
- Polonium adopts cubic structure with edge length of cube being 0.336 n...
Text Solution
|
- CsCl has bcc structure with Cs^(+) at the centre and Cl^(-) ion at eac...
Text Solution
|