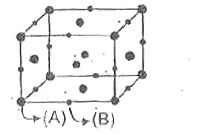
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - B) (OBJECTIVE TYPE QUESTION)|25 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - C) (PREVIOUS YEARS QUESTION)|41 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|30 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J)|10 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THE SOLID STATE -Assignment (SECTION - A) (OBJECTIVE TYPE QUESTION)
- What is the volume of a face centred cubic unit cell, when its density...
Text Solution
|
- The number of octahedral sites in a cubical close pack array of N sphe...
Text Solution
|
- For a solid with the following structure, the coordination number of t...
Text Solution
|
- The empty space between the shaded balls and hollow balls as shown in ...
Text Solution
|
- A mineral having the formula AB(2), crystallises in the cubic close -p...
Text Solution
|
- KF has NaCl type of structure. The edge length of its unit cell has be...
Text Solution
|
- Which of the following features is false regarding the structure of Cs...
Text Solution
|
- Which one has the highest melting point ?
Text Solution
|
- The mass of unit cell of Na(2)O is
Text Solution
|
- In normal spinel structure there is a closed packed array of O^(2-) io...
Text Solution
|
- The C - C and Si - C interatomic distances are 154 pm and 188 pm. The ...
Text Solution
|
- What is the coordination number of Rb^(+) in RbBr unit cell if ionic r...
Text Solution
|
- A crystalline solid AB has NaCl type structure with radius of B^(-) io...
Text Solution
|
- Number of formula units in unit cell of MgO (rock salt), ZnS (zinc ble...
Text Solution
|
- An element crystallises in a b.c.c. lattice. Nearest and next nearest ...
Text Solution
|
- The total number of elements of symmetry in a cubic crystal is
Text Solution
|
- A crystal may have one or more planes and one or more axes of symmetry...
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- Pyroelectric crystals feeble electric current
Text Solution
|
- Why does white zinc oxide on heating become yellow ?
Text Solution
|