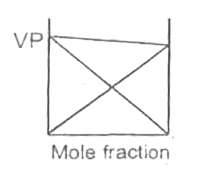
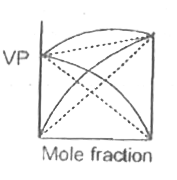
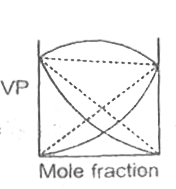
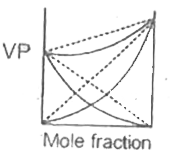
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- IF C2H5 OH and H2O solution is example of non-ideal solution then whic...
Text Solution
|
- A pair of linear equations has no solution then the graphical represen...
Text Solution
|
- If C2H5O H and H2 solution is example of non-ideal solution then whi...
Text Solution
|
- HI solution is titrated with NaOH conductometrically, graphical repres...
Text Solution
|
- IF C2H5 OH and H2O solution is example of non-ideal solution then whic...
Text Solution
|
- What is the molality of C2H5 OH in water solution which will freeze at...
Text Solution
|
- What are non-ideal solution ? Give example.
Text Solution
|
- দুটি আদর্শ ও দুটি অনাদর্শ দ্রবণের উদাহরণ দাও।
Text Solution
|
- Give one example each for non-ideal solution showing positive deviatio...
Text Solution
|