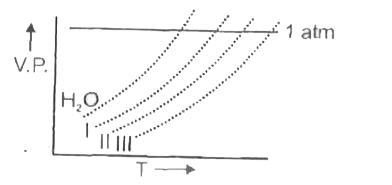
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which is having highest elevation in boiling point?
Text Solution
|
- Which of the following solutions will have highest boiling point:
Text Solution
|
- The elevation in boiling point would be highest for
Text Solution
|
- Which compound will have highest boiling point ?
Text Solution
|
- Which of the following solutions will exhibit highest boiling point?
Text Solution
|
- Which of the following solution will have highest boiling point ?
Text Solution
|
- Which of the following is expected to have highest boiling point ?
Text Solution
|
- Which is having highest elevation in boiling point?
Text Solution
|
- निम्नलिखित में से किस पदार्थ का क्वथनांक सबसे अधिक होगा
Text Solution
|