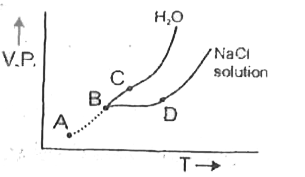
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Freezing point of solution is marked as
Text Solution
|
- Freezing point of a solution is smaller than freezing point of a solve...
Text Solution
|
- शक़्कर के 5 % ( द्रव्यमान ) जलीय विलयन का हिमांक 271K है । यदि शुद...
Text Solution
|
- At freezing point of a solution there is always
Text Solution
|
- Solution with highest freezing point is
Text Solution
|
- किसी विलयन के हिमांक को परिभाषित कीजिए ।
Text Solution
|
- घोल का हिमांक, घोलक के हिमांक से कम होता है .
Text Solution
|
- कथन-हिमांक वह तापक्रम है, जिस पर ठोस विलयन से क्रिस्टलित होते है . क...
Text Solution
|
- The freezing point of the solution M is -
Text Solution
|