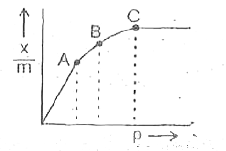
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - B (Previous year questions)|14 VideosSURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - B (Questions asked prior to medical Ent . Exams . 2005)|10 VideosSURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Exercise|20 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -J) Aakash Challengers Questions|12 VideosTEST 1
AAKASH INSTITUTE ENGLISH|Exercise EXAMPLE|134 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-SURFACE CHEMISTRY -Assignment Section - A (Objective type questions)
- Which of the following can adsorb larger volume of hydrogen gas ?
Text Solution
|
- The process of froth floatation and chromatography are based on
Text Solution
|
- The graph plotted against adsorption versus pressure P at constant tem...
Text Solution
|
- The intercept on Y - axis in the graph of log (x)/(m) versus log P g...
Text Solution
|
- Which of the following is correct about the adsorption of N(2) over l...
Text Solution
|
- By plotting log x/m on y-axis and log P on x-axis we s hould get
Text Solution
|
- In adsorption from solution phase, the Freundlich adsorption isotherm ...
Text Solution
|
- The size of colloidal particles ranges between
Text Solution
|
- Which is not a colloidal solution ?
Text Solution
|
- Lyophobic colloids are :-
Text Solution
|
- Which of the following processess best describes the purification of m...
Text Solution
|
- Colloidal solution commonly used in treatment of eye disease is :
Text Solution
|
- Micelles formation takes place
Text Solution
|
- Which of the following is positively charged colloidal particle ?
Text Solution
|
- Colloidal can be purified by
Text Solution
|
- Which of the following has minimum protecting power ?
Text Solution
|
- Migration of colloidal particles under the influence of electrie field...
Text Solution
|
- An emulsifier is an agent which:
Text Solution
|
- Gelatin is often used as an ingredient in the manufacture of ice -crea...
Text Solution
|
- Milk can be preserved by adding a few drops of
Text Solution
|