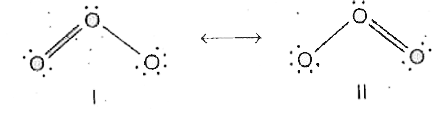A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- O(3) molecule is a resonance hybrid of the two structures I and II . ...
Text Solution
|
- Assertion: The O - O bond lengths in ozone molecule are intermediate b...
Text Solution
|
- The two oxygen-oxygen bond lengths in ozone are
Text Solution
|
- Oxygen molecule has …………… bond between two oxygen atoms .
Text Solution
|
- कथन : ओजोन में O-O आबन्ध की लम्बाई आणविक ऑक्सीजन में O-Oआबन्ध लम्बाई क...
Text Solution
|
- Oxygen molecule has ………. bond between two oxygen atoms.
Text Solution
|
- Write the Lewis dot structure of (i)Oxygen molecule (O(2)) (ii) Ethyne...
Text Solution
|
- निम्न के कारण बताएँ (i) ओजोन, ऑक्सीजन से अधिक क्रियाशील है। (ii) ऑक्स...
Text Solution
|
- ओजोन अण के ऑक्सीजन परमाण पर किस प्रकार का संकरण
Text Solution
|