A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
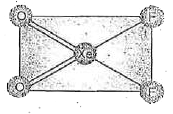
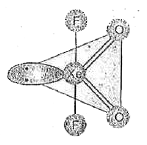
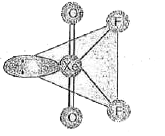
- Structure of XeO(2)F(2) is correctly represented by
Text Solution
|
- Draw the molecule structures of XeF(2),XeF(4) and XeO(2)F(2) indicatin...
Text Solution
|
- The shapes of XeO(2)F(2) molecule is
Text Solution
|
- The structure of XeO(2)F(2) is
Text Solution
|
- Structure of XeO(2)F(2) is correctly represented by
Text Solution
|
- Which of the following number of lone pair at central atom zero. XeO...
Text Solution
|
- XeO(2)F(2) is obtained by partial hydrolysis of
Text Solution
|
- The shape of XeO(2)F(2) molecule is
Text Solution
|
- The shapes of XeO(2)F(2) molecule is
Text Solution
|