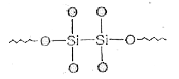
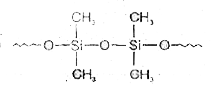
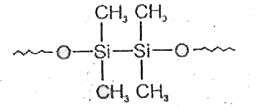
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrolysis of dimethyldichloro silane , (CH(3))(2) SiCl(2) followed by...
Text Solution
|
- Assertion Hydrolysis of (CH(3))-(2)SiCl(2) results in linear chain sil...
Text Solution
|
- (CH(3))(2) SiCl(2) on hydrolysis and on subsequent polymerisation will...
Text Solution
|
- Which of the following polymers is prepared by condensation polymerisa...
Text Solution
|
- Which of the following polymers is prepared by condensation polymerisa...
Text Solution
|
- Straight chain polymer is formed by hydrolysis of [x] which is tetrasu...
Text Solution
|
- Polymer obtained by condensation polymerisation is
Text Solution
|
- (CH(3))(2)SiCl(2) underboes hydrolysis but (CH(3))(2)"CC"l(2) does not...
Text Solution
|
- Polymer obtained by condensation polymerisation is:
Text Solution
|