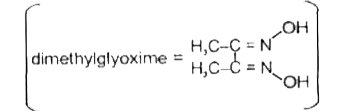A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH- ALCOHOLS, PHENOLS AND ETHERS-Assignment Section -D (Assertion - reason type question)
- Red precipitae is obtained when ethanol solution of dimethylglyoxime i...
Text Solution
|
- Assertion : Boiling point of p-nitrophenol is greater than that of o-n...
Text Solution
|
- A : When C(2) H(5) - O- CH(3) is reacted with one mole of Hl then C(2...
Text Solution
|
- A : When 3,3-dimethyl butan - 2 - ol is heated in presence of concentr...
Text Solution
|
- A : In esterification reaction , HCOOH is the most reactive acid among...
Text Solution
|
- A : Ethers can't be distilled upto dryness due to fear of explosion . ...
Text Solution
|
- Phenol is less acidic than........... .
Text Solution
|
- A : CH(3) - underset(O)underset(||)(C)-COOH gives haloform reaction ...
Text Solution
|
- A : Diphenyl ether is prepared by Williamson synthesis . R : This...
Text Solution
|
- A : Grignard's reagent is prepared in the presence of ether . R :...
Text Solution
|
- A : CH(3) - underset(CH(3))underset(|)overset(CH(3))overset(|)(C)-CH=...
Text Solution
|
- A : Two moles of Grignard reagent is consumed in the formation of ter...
Text Solution
|
- A : bond angle in ether is slightly greater than normal tetrahedral an...
Text Solution
|
- A : CH(3)-underset(CH(3))underset(|)overset(CH(3))overset(|)(C)-O-CH...
Text Solution
|
- A : Ortho - cresol is weaker acidic than meta-cresol . R : It is d...
Text Solution
|
- A : Among all ortho halophenol , fluorophenol is least acidic . R ...
Text Solution
|
- A : In esterification reaction alcohol act as nucleophile . R : ...
Text Solution
|
- A : Phenol is manufactured by Dow 's pocess. R : It involves the ...
Text Solution
|
- A : Primary alcohol is prepared by the reaction of primary amine with ...
Text Solution
|
- A : The reactivity order of alcohols is 1^(@) gt 2^(@) gt 3^(@) for ...
Text Solution
|
- A : The dehydration of ethyl alcohol in presence of Al(2)O(3) at 633 ...
Text Solution
|