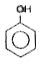
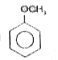
A
B
C
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(SECTION-A)|68 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(SECTION B)|6 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|28 VideosMOCK_TEST_20
AAKASH INSTITUTE ENGLISH|Exercise EXAMPLE|29 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise TRY YOURSELF|28 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES -Exercise
- Carbon-carbon bond-order in benzene is -1.5 due to
Text Solution
|
- Which of the following is the most acidic?
Text Solution
|
- In which of the following, the group attached to the benzene ring show...
Text Solution
|
- Which of the following compound can show resonance?
Text Solution
|
- Which of the following is the most stable carbocation?
Text Solution
|
- Electromeric effect involves the complete transfer of
Text Solution
|
- which of the following is most acidic in natute:
Text Solution
|
- The most deactivating group for electrophilic substitution reaction in...
Text Solution
|
- The number of alpha carbon-hydrogen atoms present in C(2)H(5)-underset...
Text Solution
|
- Hyperconjugation involves delocalisation of.....
Text Solution
|
- Which of the following alkene is the most unstable
Text Solution
|
- The maximum number of no bond resonating structure is possible in
Text Solution
|
- How many ways a bond between two atoms can cleave?
Text Solution
|
- Which of the following is the most reactive towards electrophilic subs...
Text Solution
|
- Which of the following can act as an electrophile?
Text Solution
|
- Which of the following is neutral nucleophile?
Text Solution
|
- The group which is most activating is
Text Solution
|
- Hyperconjugation is also known as
Text Solution
|
- Which of the following is electron deficient species?
Text Solution
|
- Hybridisation of carbon in CH(2)=CH-overset(o+)(C )H(2)
Text Solution
|