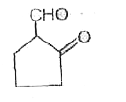
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound does not reacts with Tollen's reagent but undergoe...
Text Solution
|
- Consider the following Baeyer-Villiger oxidation.
Text Solution
|
- Select the correct Baeyer-Villiger oxidation reaction:
Text Solution
|
- A compound of the formula C(4)H(10)O reacts with sodium and undergoes ...
Text Solution
|
- An organic compound does not react appreciably with Lucas reagent but ...
Text Solution
|
- The compound of molecular formula C(5)H(10)O (A) reacts with Tollen's ...
Text Solution
|
- An organic compound does not react appreciably with Lucas reagent but ...
Text Solution
|
- The compound which reacts with hydroxylamine but does not react with T...
Text Solution
|
- Which of the following compound does not react with Tollen's reagent?
Text Solution
|