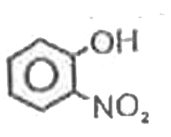
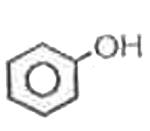
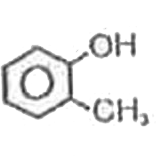
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - C (Questions asked Prior to medical Ent. Exams. 2005)|13 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -D (Assertion - reason type question)|20 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - B (Objective type questions)|25 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH- ALCOHOLS, PHENOLS AND ETHERS-Assignment Section - C (Previous Years questions)
- Which of the following is not the product of dehydration of
Text Solution
|
- Which of the following reaction(s) can be used for the preparation of ...
Text Solution
|
- The reaction CH(3)-underset(CH(3))underset(|)overset(CH(3))overset(|...
Text Solution
|
- Among the following sets of reactants which one produces anisole?
Text Solution
|
- Identify Z in the sequence of reactions : CH(3)CH(2)CH=CH(2)underse...
Text Solution
|
- Among the following ethers, which one will produce methyl alcohol on t...
Text Solution
|
- In the following reaction The major product is
Text Solution
|
- In the following reactions . ltb rgt CH3-overset(CH3)overset(|)CH-unde...
Text Solution
|
- Which one of the following compounds has the most acidic nature ?
Text Solution
|
- Which one of the following is most reactive towards electrophillic rea...
Text Solution
|
- Among the following four compounds (a) Phenol (b) methyl phenol ...
Text Solution
|
- When glycerol is treated with excess of HI, it produces -
Text Solution
|
- Which one of the following compounds will be most readily dehydrated?
Text Solution
|
- Which of the following conformers for ethylene glycol is most stable ?
Text Solution
|
- Consider the following reaction "Ethanol" overset(PBr(3))to X overset...
Text Solution
|
- Consider the following reaction : Phenol underset(Zn" dust")toXun...
Text Solution
|
- H(2)COH.CH(2)OH on heating with periodic acid gives :
Text Solution
|
- The major organic product in the reaction CH(3)-O-CH(CH(3))(2)+HIrar...
Text Solution
|
- Ethylene oxide when treated with Grignard reagent yields
Text Solution
|
- Which one of the following compounds is most acidic
Text Solution
|